Activation of Photo-Fenton-like Process Catalyzed by Fe3+-IDS under Visible LED Light Irradiation
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
This study is focused on the effect of the light source (LEDs emitting visible and UV-A light) on the activation of photo-Fenton processes for the Fe3+-iminodisuccinic acid/H2O2 catalytic system. The catalyst enables visible light for the hydroxyl radical’s formation by H2O2 conversion. This study shows, for the first time, the central role of visible light to accelerate the process kinetics favoring the complete degradation of a probe molecule ([gallic acid] = 25.0 mg L–1 (0.147 mM)), in water solutions in 90 min using a [H2O2]/catalyst molar ratio (R) of 75, in comparison with data obtained under dark conditions (99% under visible radiation vs 67% in dark conditions). A kinetic model able to correlate the electrical nominal power of the used LEDs, the light absorption properties of Fe3+-iminodisuccinic acid (Fe-IDS), and LEDs emission spectra was developed to estimate the degradation kinetic constants for the process in dark conditions and in the presence of light. The accuracy of the calculated kinetic constants was tested under different R values to verify the predictive ability of the model. A very good agreement between the experimental data and the mathematical model calculations was achieved. These results highlight the promising use of the Fe-IDS catalyst for environmental remediation considering low energy consumption conditions. A reasonable explanation is that the presence of Fe-IDS significantly promoted the generation of reactive oxygen species using visible light and H2O2, which led to gallic acid removal.
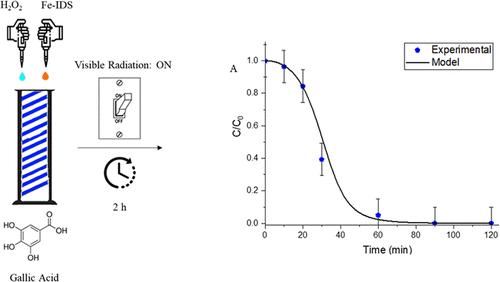
可见光LED照射下Fe3+-IDS催化的类光芬顿过程的活化研究
本研究的重点是光源(发光二极管发射可见光和UV-A光)对Fe3+-亚氨基二丁二酸/H2O2催化体系的光- fenton过程的激活的影响。催化剂为H2O2转化形成羟基提供可见光。该研究首次表明,在[H2O2]/催化剂摩尔比(R)为75的情况下,与在黑暗条件下获得的数据(可见光下99%,黑暗条件下67%)相比,可见光对加速过程动力学的核心作用有利于探针分子([没食子酸]= 25.0 mg L-1 (0.147 mM))在90分钟内在水溶液中完全降解。建立了一个动力学模型,该模型能够将所使用led的标称电功率、Fe3+-亚氨基二丁二酸(Fe-IDS)的光吸收特性和led发射光谱相关联,以估计该过程在黑暗条件下和有光存在下的降解动力学常数。在不同的R值下,对计算得到的动力学常数的准确性进行了测试,验证了模型的预测能力。实验数据与数学模型计算结果吻合较好。这些结果突出了Fe-IDS催化剂在低能耗条件下的环境修复应用前景。一个合理的解释是,Fe-IDS的存在显著促进了可见光和H2O2产生活性氧,从而导致没食子酸的去除。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


