Selective Hydroformylation of 1,3-Butadiene to Adipic Aldehyde Enabled by a Ligand-Relay Catalysis
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The hydroformylation of 1,3-butadiene for the selective synthesis of adipic aldehyde (AA) has been a long-standing challenge due to its intricate reaction network, resulting in low reaction efficiency and poor chemo- and regioselectivity. Herein, we propose a dual ligand-relay catalysis strategy for the selective hydroformylation of 1,3-butadiene to an AA. This strategy entails a one-pot and two-step process, commencing with a Rh-catalyzed 1,3-butadiene hydroformylation, followed by Rh-catalyzed isomerizing hydroformylation. The reaction is facilitated by using two distinct biphosphites as auxiliary ligands, each with a specific individual role in the process. Remarkably, an unprecedented chemo- and regioselectivity of up to 65.9% toward AA was achieved with complete conversion of 1,3-butadiene, marking the highest value compared to the previous state-of-the-art catalyst systems thus far. Furthermore, the process also produced a 26.5% selectivity for n-valeraldehyde as the major byproduct, a key compound of industrial importance. This study therefore represents a significant advancement in the hydroformylation of 1,3-butadiene, showcasing the potential of this ligand-relay catalysis strategy for improving selectivity in the reaction.
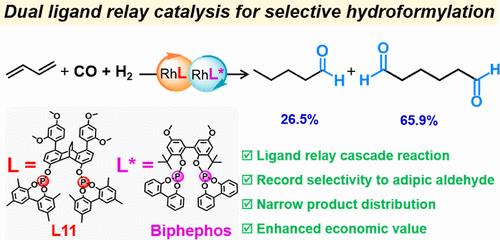
配体接力催化1,3-丁二烯选择性氢甲酰化制备己二醛
1,3-丁二烯氢甲酰化选择性合成己二醛(AA)由于其复杂的反应网络,导致反应效率低,化学选择性和区域选择性差,长期以来一直是一个挑战。在此,我们提出了一种双配体接力催化策略,用于1,3-丁二烯选择性氢甲酰化为AA。这一策略需要一个一锅两步的过程,从铑催化的1,3-丁二烯氢甲酰化开始,然后是铑催化的异构化氢甲酰化。通过使用两种不同的二磷酸盐作为辅助配体,促进了反应,每种配体在过程中都具有特定的个体作用。值得注意的是,在1,3-丁二烯完全转化的情况下,对AA的化学选择性和区域选择性达到了前所未有的65.9%,这是迄今为止与之前最先进的催化剂体系相比的最高值。此外,该工艺对主要副产物n-戊醛的选择性为26.5%,这是工业上重要的关键化合物。因此,这项研究代表了1,3-丁二烯氢甲酰化的重大进展,展示了这种配体接力催化策略在提高反应选择性方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


