Dual BACH1 regulation by complementary SCF-type E3 ligases
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
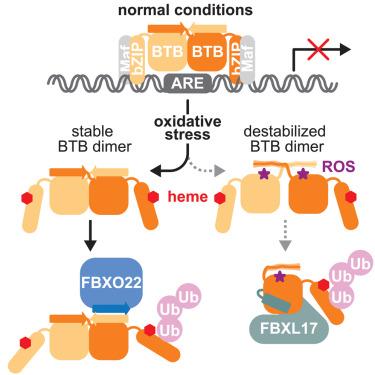
Broad-complex, tramtrack, and bric-à-brac domain (BTB) and CNC homolog 1 (BACH1) is a key regulator of the cellular oxidative stress response and an oncogene that undergoes tight post-translational control by two distinct F-box ubiquitin ligases, SCFFBXO22 and SCFFBXL17. However, how both ligases recognize BACH1 under oxidative stress is unclear. In our study, we elucidate the mechanism by which FBXO22 recognizes a quaternary degron in a domain-swapped β-sheet of the BACH1 BTB dimer. Cancer-associated mutations and cysteine modifications destabilize the degron and impair FBXO22 binding but simultaneously expose an otherwise shielded degron in the dimer interface, allowing FBXL17 to recognize BACH1 as a monomer. These findings shed light on a ligase switch mechanism that enables post-translational regulation of BACH1 by complementary ligases depending on the stability of its BTB domain. Our results provide mechanistic insights into the oxidative stress response and may spur therapeutic approaches for targeting oxidative stress-related disorders and cancer.
互补的scf型E3连接酶对BACH1的双重调控
Broad-complex, tramtrack,和bric-à-brac结构域(BTB)和CNC同源物1 (BACH1)是细胞氧化应激反应的关键调控因子,也是一种受两种不同的F-box泛素连接酶SCFFBXO22和SCFFBXL17严格控制的癌基因。然而,这两种连接酶在氧化应激下如何识别BACH1尚不清楚。在我们的研究中,我们阐明了FBXO22识别BACH1 BTB二聚体的结构域交换β-片中的四元结构的机制。癌症相关的突变和半胱氨酸修饰破坏了degron的稳定性,破坏了FBXO22的结合,但同时暴露了二聚体界面中一个被屏蔽的degron,使FBXL17能够将BACH1识别为单体。这些发现揭示了一种连接酶开关机制,这种机制使BACH1通过互补连接酶进行翻译后调节,这取决于其BTB结构域的稳定性。我们的研究结果提供了氧化应激反应的机制见解,并可能刺激针对氧化应激相关疾病和癌症的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


