The ‘Treg paradox’ in inflammatory arthritis
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
Abstract
Classic regulatory T (Treg) cells expressing CD4 and the hallmark transcription factor FOXP3 are integral to the prevention of multi-system autoimmunity. However, immune-mediated arthritis is often associated with increased numbers of Treg cells in the inflamed joints. To understand these seemingly conflicting observations, which we collectively describe as ‘the Treg paradox’, we provide an overview of Treg cell biology with a focus on Treg cell heterogeneity, function and dysfunction in arthritis. We discuss how the inflamed environment constrains the immunosuppressive activity of Treg cells while also promoting the differentiation of TH17-like Treg cell, exTreg cell (effector T cells that were formerly Treg cells), and osteoclastogenic Treg cell subsets that mediate tissue injury. We present a new framework to understand Treg cells in joint inflammation and define potential strategies for Treg cell-directed interventions in human inflammatory arthritis. In this Review, Nigrovic and colleagues examine potential mechanisms underlying the paradoxical continuation of inflammation in arthritis, despite the increased numbers of regulatory T cells in inflamed joints, and discuss the implications for regulatory T cell-targeted therapeutic interventions in inflammatory arthritis.
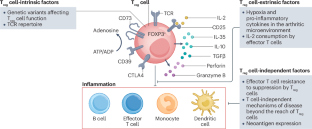

炎性关节炎中的“Treg悖论”
表达CD4和标志性转录因子FOXP3的经典调节性T (Treg)细胞是预防多系统自身免疫不可或缺的一部分。然而,免疫介导的关节炎通常与炎症关节中Treg细胞数量的增加有关。为了理解这些看似矛盾的观察结果,我们将其统称为“Treg悖论”,我们概述了Treg细胞生物学,重点关注关节炎中的Treg细胞异质性、功能和功能障碍。我们讨论了炎症环境如何限制Treg细胞的免疫抑制活性,同时也促进th17样Treg细胞、Treg细胞(以前是Treg细胞的效应T细胞)和介导组织损伤的破骨性Treg细胞亚群的分化。我们提出了一个新的框架来理解Treg细胞在关节炎症中的作用,并确定Treg细胞定向干预人类炎症性关节炎的潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


