Modeling Solubilities for Amino Acids in Water as Functions of Temperature and pH
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
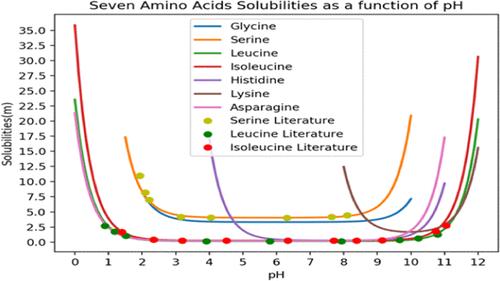
Experimental results from the literature as well as from new experimental results presented here are used to determine parameters to correlate amino acid solubilities and activities in water. In particular, we have used a modified Larsen’s UNIQUAC Functional-group Activity Coefficients (UNIFAC) group-contribution model to illustrate the solubility behavior of 10 amino acids in water including l-Histidine and l-Arginine that, to our knowledge, have not been published previously. New UNIFAC groups have been introduced to obtain the activity coefficients for the prediction of activities and solubilities as a function of temperature and pH. Prior models have not accounted for ionic interactions that affect pH dependent behavior and predictions. Hence, we examine whether a combination of UNIFAC and a new modified Debye–Huckel equation by Rapp et al.1 is able to predict the activity coefficients of ionic species in solution at the high ionic strengths seen at high and low pH. We measured solubilities and fitted binary amino acid activity coefficients to estimate the new UNIFAC interaction parameters. The newly obtained UNIFAC parameters were used for the prediction of amino acid solubilities when they were predominantly charge-neutral. Then ionic interactions and pH-dependent chemical equilibria were added to calculate amino acid solubilities in aqueous solutions at different values of pH and temperature. The chemical equilibria required were calculated in a manner similar to Visual-MINTEQ2 that will be described in a separate publication. The calculated solubilities were found to be in good agreement with our experimental measurements and with literature data.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


