Hematopoietic Stem Cell Transplantation in Patients With Myelofibrosis and Splanchnic Vein Thrombosis: A Case Series
IF 10.1
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract

骨髓纤维化和内脏静脉血栓患者的造血干细胞移植:一个病例系列
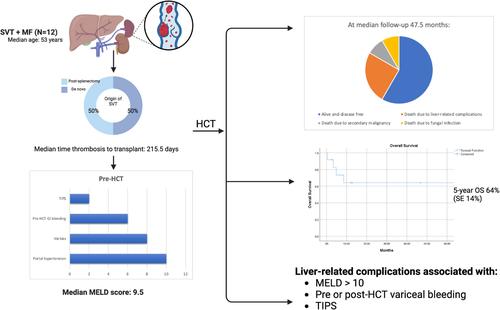
骨髓增生性肿瘤(MPN)与内脏静脉血栓形成(SVT)的风险增加有关,SVT是导致发病率和死亡率的重要因素。SVT,包括门静脉、肠系膜上静脉、脾静脉和肝静脉血栓形成,破坏门静脉压力,导致门静脉高压(PHT)的发展和并发症,如静脉曲张、脾肿大和肝功能衰竭,从而增加发病率和死亡率。既往SVT对同种异体造血干细胞移植(HCT)治疗骨髓纤维化(MF)患者预后的影响尚不清楚。虽然一个小的病例系列发现SVT和高胆红素血症[1]之间有很强的相关性,但数据有限,使得抗凝、肝脏合并症和PHT后遗症的最佳管理在这一患者群体中具有挑战性。本研究评估了先前存在的SVT及其治疗对移植结果的影响。我们筛选了2000年1月至2023年8月期间在德克萨斯大学MD安德森癌症中心连续接受HCT治疗的334例MF患者,并确定了12例先前存在SVT。通过回顾性回顾收集患者特征、肝脏和血栓相关数据、移植细节和结果。使用肌酐、胆红素、INR和钠水平计算终末期肝病模型(MELD)评分,对慢性肝功能障碍程度进行分层。连续变量报告为中位数和范围,而分类变量报告为数量和百分比。用Kaplan-Meier法估计总生存率。关于患者特征,中位年龄为53岁(范围:39-73岁)。5例为原发性MF, 4例为原发性血小板增多症(PET-MF), 3例为真性红细胞增多症(PPV-MF)。多数(n = 8, 66.6%)为JAK2 v617f阳性。1例患者MPL阳性。患者接受基于机构方案的调理方案,考虑年龄和合并症。表1总结了所有患者的个体特征和结果。其他基线特征见表S1。表1。详细的病人特征。移植时患者年龄、性别、jak2状态、通过ngs产生的额外突变血栓形成类型、移植前SVT天数、血栓形成前脾切除术天数、yerdel分类、抗凝治疗日期、移植调节方案140fpositive venen脾脏静脉血栓形成sis109noneno3 - 7月23日bu / cy270fpositive ven门静脉、SMV和脾静脉血栓形成sis145yes3yes, 120天14- 9月22日flu /Mel372MPositiveASXL1、EZH2、GATA2、门静脉血栓形成、门静脉血栓形成、脾静脉血栓形成、门静脉血栓形成、脾静脉血栓形成、门静脉血栓形成、脾静脉血栓形成、门静脉血栓形成、脾静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成、门静脉血栓形成门静脉分流15- 12月9日flu /Bu/ thiotepa1033mnee门静脉血栓形成46yes3yes, 95天24- 10月5日flu /Bu/ thiotepa1162mnee门静脉血栓形成197yes1yes, 125天与tips9 - 4月3日flu /Bu/Thiotepa1249FNENESMV及脾静脉血栓形成;患者供体类型eld评分门静脉高压腹水静脉曲张移植前静脉曲张出血±干预使用β受体阻滞剂移植后静脉曲张出血前100天肝脏结局临床病程1 haplo8 yes2食管noyes3、4、11天主要胃肠道出血,需要脾动脉栓塞无并发症2 mud8 yes2食管nono1无并发症3 msd7 yes2食管严重的高胆红素血症和VOD(第4天)失代偿性肝功能衰竭合并脑病(VOD)、血小板难治性和颅内出血(第6天)食管、胃no,先发制人绑扎(第13天)轻微胃肠道出血(第1386天)胃、脾静脉曲张及脾大7mmud17 yes多发性腹水旁腔食管yes,静脉曲张绑扎yes胃肠道大出血第12、68、84、105、111天I级高胆红素血症,需要反复引流的中度转氨性胸膜积液,进行性肾衰竭及真菌性肺炎所致死亡8msd13 yes,需要穿刺食管yes第14、3223天复发性胃肠道大出血I级高胆红素血症代偿性肝硬化需要穿刺,肝脏移植物抗宿主病。 复发日2549msd9non - ononononadadnon - onononadadnon - I级转氨炎死于继发性恶性肿瘤11msd10yesyesnoyes,静脉曲张束带non - onad复发性脑病和腹水需要行TIPS治疗12msd13yesyes,需要行食管、胃旁穿刺;复发性腹水和脑病需要行TIPS治疗,并发严重的消化道出血伴凝血功能障碍;一半的患者在脾切除术后发展为上室t,而另一半则发展为新的上室t。3例在hct前100天内发生SVT,均为脾切除术后。从血栓形成到移植的中位时间为215.5天(范围:41-4748天)。描述血栓形成程度和并发症风险的Yerdel评分中位数为3(范围:1-3)。6例(50%)患者患有慢性上室血栓,hct前未进行抗凝治疗。其余6例(50%)因急性血栓而接受抗凝治疗(DOAC或依诺肝素),中位持续时间为180天(95-1950)。3例(42.8%)在血小板恢复后继续抗凝。进一步的信息见表S2。关于肝脏相关特征,MELD中位评分为9.5(范围:8-17)。大多数患者有PHT的放射学证据(n = 10;83.3%)或静脉曲张(n = 8;66.6%)。在静脉曲张患者中,6例(75%)在hct前有静脉曲张出血。其中2人接受静脉曲张捆扎,4人接受受体阻滞剂预防。2例患者需要hct前介入经颈静脉肝内门静脉系统分流术(TIPS)和门静脉分流术。hct前未发现活检证实的肝硬化或铁超载,尽管只有4/12患者进行了活检。没有发现肝功能障碍的其他原因。表S3显示了更多信息。在第100天,11例(92%)患者存活且无病。在中位47.5个月的随访中,7例(66.6%)存活且无病。5年总生存率为64% (SE为14%)。5例患者死亡:1例死于继发性恶性肿瘤(2315天),1例死于与移植物功能不良相关的真菌感染(148天),尽管复发性胃肠道(GI)出血和腹水导致了他们的死亡,3例死于直接与肝脏相关的并发症(静脉闭塞性疾病[VOD]和颅内出血,第12天;第103天和第260天肝功能衰竭)。对于那些直接死于肝脏相关并发症的患者:第一位患者(MELD 15, hct前静脉曲张出血需要绑扎)在hct后第4天出现严重的VOD,并在第12天死于颅内出血。第二例患者(MELD 10, hct前静脉曲张出血需要绑扎)在第62天因门静脉和脾静脉血栓植入TIPS后出现肝功能衰竭,导致第103天死亡。第三例患者(MELD 13) hct后出现症状性腹水,影像学显示急性肝静脉血栓(BCS)和海海绵门静脉血栓,需要在第95天放置TIPS。她出现了进行性肝功能衰竭和胃肠道出血,于第260天死亡。总体而言,肝脏相关并发症与hct前MELD评分[gt; 10]、hct前或hct后静脉曲张出血、TIPS放置和hct后成像再通失败有关。非致命性肝脏相关并发症包括肝毒性和进行性血栓形成。1级高胆红素血症(n = 3;25%)和1级转氨炎(n = 2;17%)在没有干预的情况下在第100天消退。5名患者(42%)在中位146.5天(范围:72-3304天)出现了PHT的长期后遗症。其中包括失代偿性腹水(n = 3;33.3%),静脉曲张出血需要内镜治疗(n = 4;33.3%)和脑病(n = 2;16%)。脾脏中位大小为24cm, 75%未行脾切除术。hct后复发性胃肠道出血(中位76天,范围:3-3323)发生在hct前食管静脉曲张且未抗凝的患者中,尽管有阻断剂预防。4例hct后患者出现新的静脉血栓栓塞(VTE),其中3例(75%)发生在停用抗凝治疗期间。其中3例(75%)发生在内脏系统外(四肢深静脉和肺栓塞)。SVT再通仅发生在16%的患者中。尽管如此,一些患者在延长随访时有令人鼓舞的临床结果。患者8 (MELD 8)在hct后9年复发性静脉曲张出血和腹水,需要穿刺,但在hct后12.5年存活且临床稳定,并进行了长期肝病随访和利尿剂治疗。患者6 (MELD 9)在hct后出现静脉曲张增加和脾肿大,但在hct后2577天无疾病,无肝毒性。这一系列的病例表明,尽管MF患者面临肝脏和血栓形成相关的发病率和死亡率,但已有SVT的患者可以成功地进行HCT。 SVT的最佳管理,特别是在hct期,还没有很好的研究。然而,在这种情况下,血栓复发率增加了100。虽然抗凝治疗急性血栓可以改善再通和减少PHT,但它可能会增加出血,特别是在静脉曲张的情况下。考虑β阻断或先发制人的绑扎可以减轻这种风险,尽管这尚未在MF中得到验证。慢性上室血栓很少再通,因此通常建议终身抗凝以降低复发风险。在我们的队列中,长期抗凝,包括围hct,与出血无关,尽管继续抗凝围hct的决定应该个体化。在非hct患者中,TIPS、溶栓和肝移植等干预措施的结果是不同的。门静脉系统分流对BCS患者的生存有好处,但对SVT患者的益处尚不清楚。脾肿大在hct术前和术后的处理具有挑战性。脾切除术常用于逆转PHT和改善植入术,然而腹腔镜入路术后SVT发生率高达55%。发病率可能很高,包括肠缺血甚至死亡。发生SVT的危险因素包括脾重、同时存在的MPN、脾/门静脉直径、低白细胞计数和解剖变异[5]。在我们的队列中,有一半的患者在脾切除术后发生了SVT,但脾切除术本身似乎与长期发病率无关。由于铁超载、髓外造血和先前存在的PHT, MF患者存在hct后肝毒性的风险。Wong等人发现,在因MF而接受HCT的患者中,VOD的风险增加,SVT与高胆红素血症[1]之间存在关联。相比之下,我们的数据显示100天后没有持续的肝功能障碍,这表明通过适当的患者选择,肝功能不会受到损害。1例患者死于VOD并发症,表明在hct后时期需要仔细监测。PHT是MF的常见并发症,其潜在病因包括脾肿大、PVT、髓外造血以及最近的窦性纤维化。由此产生的静脉曲张增加了出血和腹水的风险,导致长期发病。血栓形成往往是原因之一,在随访期间,近一半的JAK2 v617f相关非肝硬化PVT的非hct患者发生PHT。在我们的队列中,PHT的后遗症在第100天就很明显,有些患者甚至在12年后仍有明显的持续发病率。肝脏相关变量如胆红素、白蛋白和INR、MELD评分(> 10)和非再通hct前是该队列的关键预后指标。其他高风险临床特征,包括hct前静脉曲张出血,似乎可以预测hct后类似的出血,无论干预措施如何。hct前的优化,如静脉曲张绑扎,可能会改善结果,尽管这一点尚不清楚。所有接受HCT检查的患者都应该有专门的PHT成像、静脉曲张治疗的内窥镜评估和长期的多学科随访。虽然我们的研究受到患者队列小、回顾性设计和缺乏BCS或肝静脉血栓代表的限制,但表明HCT对于早期和总死亡率低的选定的SVT患者是可行的和可治愈的。然而,PHT的长期后遗症仍然令人担忧。静脉曲张出血和MELD评分10等因素似乎会增加与PHT相关的未来发病率和死亡率的风险。多学科的hct前评估,包括PHT的专用成像,静脉曲张的筛查和预防性治疗,以及长期随访有助于该患者群体的成功结果。需要更大规模的研究来验证这些结果,并对接受HCT的SVT患者进行更精细的风险分层。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
15.70
自引率
3.90%
发文量
363
审稿时长
3-6 weeks
期刊介绍:
The American Journal of Hematology offers extensive coverage of experimental and clinical aspects of blood diseases in humans and animal models. The journal publishes original contributions in both non-malignant and malignant hematological diseases, encompassing clinical and basic studies in areas such as hemostasis, thrombosis, immunology, blood banking, and stem cell biology. Clinical translational reports highlighting innovative therapeutic approaches for the diagnosis and treatment of hematological diseases are actively encouraged.The American Journal of Hematology features regular original laboratory and clinical research articles, brief research reports, critical reviews, images in hematology, as well as letters and correspondence.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


