Development of a New Vaccine Adjuvant System Based on the Combination of the Synthetic TLR4 Agonist FP20 and a Synthetic QS-21 Variant
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
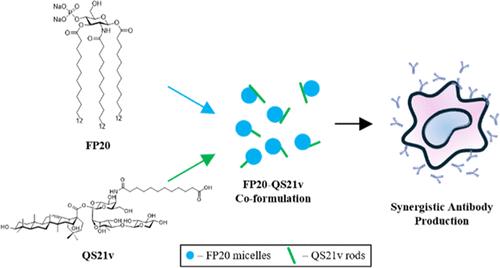
In this study, we formulated an alternative to AS01b by combining FP20, a synthetic TLR4 agonist, and QS21v, a minimal saponin adjuvant, aiming to improve the vaccine efficacy and stability. The phase transition temperature of FP20 was determined by using differential scanning calorimetry to be 43.9 °C, providing a foundation for the formulation process. The coformulation was prepared using a dry film method for even adjuvant distribution. Characterization by dynamic light scattering and nanoparticle tracking analysis revealed a uniform particle size distribution of ∼120 nm. Cryogenic electron microscopy (CryoEM) revealed nanosized interactions between FP20 and QS21v, forming stable structures that likely enhanced the antigen presentation and immune activation. These physicochemical properties contributed to a robust in vivo synergy, where the coformulation elicited significantly higher antigen-specific antibody titers compared to individual adjuvants. These findings suggest that the FP20+QS21v coformulation provides a potent, stable, and safer alternative to traditional adjuvants, enhancing both vaccine efficacy and immunogenicity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


