Rapid Synthesis of Defect-Free Tubular Co-Gallate MOF Membranes for MeOH/MTBE Separation by Pervaporation
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
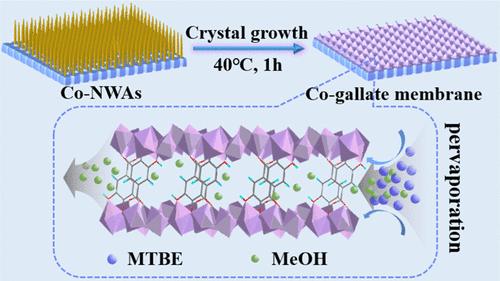
The Co-gallate MOF membrane exhibits great potential for separating MeOH/MTBE mixtures due to its regular pore size, exceptional stability, and high hydrophilicity. Nevertheless, the rapid nucleation and growth rate of the Co-gallate crystals in the synthesis solution cause poor nucleation on the substrate surface, which presents a significant challenge for the preparation of continuous Co-gallate membranes. In this study, we presented a cobalt carbonate hydroxide nanowire array (Co-NWA)-induced strategy that enabled the rapid and defect-free synthesis of tubular Co-gallate membranes within just 1 h. The presynthesized Co-NWAs on the substrate played as nucleation centers and anchoring sites, facilitating robust membrane formation. The effects of various synthesis parameters on the crystal morphology and membrane compactness were systematically examined. The optimized Co-gallate membrane demonstrated excellent pervaporation performance, with a permeation flux of 2.03 kg m–2 h–1 and a separation factor of 6711 for a 14.3/85.7 wt % MeOH/MTBE mixture, maintaining high performance for over 100 h, indicative of its remarkable long-term stability. This Co-NWA-induced synthesis strategy presents a promising approach for the industrial-scale application of Co-gallate MOF membranes and the design of other MOF membranes, thereby advancing their utility in requisite liquid separation processes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|---|---|
| 阿拉丁 |
3-Aminopropyltriethoxysilane (APTES)
|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


