Catalytic Enantioselective C–C Coupling of Alcohols for Polyketide Total Synthesis beyond Chiral Auxiliaries and Premetalated Reagents
IF 51.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Catalytic enantioselective hydrogen autotransfer reactions for the direct conversion of lower alcohols to higher alcohols are catalogued and their application to the total synthesis of polyketide natural products is described. These methods exploit a redox process in which alcohol oxidation is balanced by reductive generation of organometallic nucleophiles from unsaturated hydrocarbon pronucleophiles. Unlike classical carbonyl additions, premetalated reagents, chiral auxiliaries and discrete alcohol-to-aldehyde redox reactions are not required. Additionally, chemoselective dehydrogenation of primary alcohols in the presence of secondary alcohols enables C–C coupling in the absence of protecting groups.
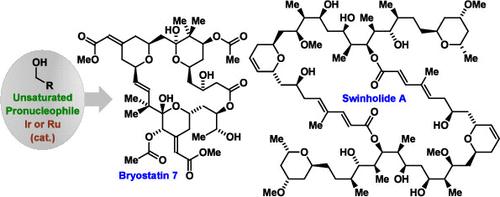
醇类催化对映选择性C-C偶联在手性助剂和预金属试剂外的聚酮总合成中的应用
对低醇直接转化为高醇的催化对映选择性氢自转移反应进行了分类,并介绍了其在聚酮类天然产物全合成中的应用。这些方法利用一种氧化还原过程,其中酒精氧化是通过不饱和烃亲核试剂还原生成有机金属亲核试剂来平衡的。与传统的羰基添加物不同,不需要预金属试剂、手性助剂和离散醇制醛的氧化还原反应。此外,伯醇在仲醇存在下的化学选择性脱氢使C-C偶联在没有保护基团的情况下成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


