Synergistic mechanism of disparate surface hydroxyls and oxygen vacancies towards peroxymonosulfate activation for durable water decontamination
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Constructing oxygen vacancies (OVs) and surface hydroxyls are efficient methods in catalyst modification engineering. However, their unclear interrelationships limit the design of more efficient catalysts with dual active centers. For this purpose, we concurrently constructed the OVs and surface hydroxyls by Ni2+ doping α-FeOOH. The isomorphic substitution of Ni2+ to Fe3+ induced remarkable formation of OVs with the crystalline phase transformed to α-Fe2O3 and partial structure hydroxyls synchronously preserved. α-Fe1.6Ni0.4O3H showed better performance in activating peroxymonosulfate (PMS) to degrade clothianidin (CLO) than α-FeOOH and α-Fe2O3, and almost complete removal of CLO within 30 min was achieved. Various in situ experiments and DFT calculations demonstrated that the hydrolyzed hydroxyls were prone to fill the OVs, leading to the poisoning of active sites, and replacing it is the necessary pathway for surface-complexation of PMS. The structure hydroxyls were verified to alleviate the occupation of hydrolyzed hydroxyls on OVs by hydrogen-bonding interaction, thus forming sustainable OVs and enhancing the PMS adsorption. Besides, OVs and structure hydroxyls synergistically regulated the spin state and enhanced the electron transfer ability of surrounding Fe sites, promoting PMS activation. Furthermore, α-Fe1.6Ni0.4O3H/PMS system has good environmental tolerance, especially for common oxygenated anions (SO42-, NO3– and HCO3–). Coupling with membrane filtration further improved its practicality, and the coupling system achieved excellent removal of CLO, UV254 and DOM in raw water and filtered water. These findings lay a foundation for identifying the role of OVs and surface hydroxyls, as so to give new inspiration for developing efficient catalysts to facilitate PMS activation, and also propose a superior strategy for practical application of the catalytic system in water treatment.
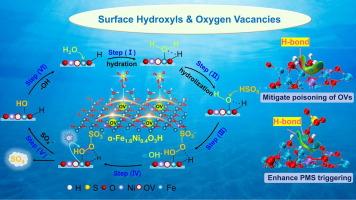
不同表面羟基和氧空位对过氧单硫酸盐活化持久水去污的协同机制
构建氧空位(OVs)和表面羟基是催化剂改性工程中的有效方法。然而,它们之间不明确的相互关系限制了具有双活性中心的更有效催化剂的设计。为此,我们用Ni2+掺杂α-FeOOH同时构建了OVs和表面羟基。Ni2+与Fe3+的同构取代导致OVs的形成,晶相转变为α-Fe2O3,部分结构羟基同步保留。α-Fe1.6Ni0.4O3H对过氧单硫酸盐(PMS)的降解效果优于α-FeOOH和α-Fe2O3,在30 min内几乎完全去除cloo。各种原位实验和DFT计算表明,水解羟基容易填充OVs,导致活性位点中毒,取代它是PMS表面络合的必要途径。结构羟基通过氢键相互作用减轻了水解羟基在OVs上的占据,从而形成可持续的OVs,增强了PMS的吸附。此外,OVs和结构羟基协同调节了自旋状态,增强了周围Fe位点的电子转移能力,促进了PMS的活化。α-Fe1.6Ni0.4O3H/PMS体系具有良好的环境耐受性,特别是对常见的氧阴离子(SO42-、NO3 -和HCO3 -)耐受性较好。与膜过滤的耦合进一步提高了其实用性,耦合系统对原水和过滤水中的CLO、UV254和DOM均有较好的去除效果。这些发现为进一步明确OVs和表面羟基的作用奠定了基础,为开发促进PMS活化的高效催化剂提供了新的灵感,也为催化体系在水处理中的实际应用提出了更好的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


