Cancer-associated fibroblasts regulate mitochondrial metabolism and inhibit chemosensitivity via ANGPTL4-IQGAP1 axis in prostate cancer
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Cancer-associated fibroblasts (CAFs) are a critical component of the tumor microenvironment, being implicated in enhancing tumor growth and fostering drug resistance. Nonetheless, the mechanisms underlying their function in prostate cancer (PCa) remain incompletely understood, which is essential for devising effective therapeutic strategies.Objectives
The main objective of this study was to explore the mechanisms by which CAFs mediate PCa growth and chemoresistance.Methods
We validated through data analysis and experimentation that CAFs significantly impact PCa cell proliferation and chemoresistance. Subsequently, we conducted a comprehensive proteomic analysis of the conditioned media from CAFs and PCa cells and identified angiopoietin-like protein 4 (ANGPTL4) as a key factor. We employed ELISA and multiplex immunofluorescence assays, all of which indicated that ANGPTL4 was primarily secreted by CAFs.Next, we conducted metabolomics analysis, GST pull-down assays, Co-IP, and other experiments to explore the specific molecular mechanisms of ANGPTL4 and its precise effects on PCa cells. Through drug screening, we identified Quercetin 3-O-(6′-galactopyranosyl)-β-D-galactopyranoside (QGGP) as an effective inhibitor of CAFs function. Finally, we thoroughly assessed the therapeutic potential of QGGP both as a monotherapy and in combination with docetaxel in PCa cellsResults
We discovered that the extracrine factor ANGPTL4 is primarily expressed in CAFs in PCa. When ANGPTL4 binds to IQ motif-containing GTPase-activating protein 1 (IQGAP1) on the PCa cell membrane, it activates the Raf-MEK-ERK-PGC1α axis, promoting mitochondrial biogenesis and OXPHOS metabolism, and thereby facilitating PCa growth and chemoresistance. Furthermore, virtual and functional screening strategies identified QGGP as a specific inhibitor of IQGAP1 that promotes its degradation. Combined with docetaxel treatment, QGGP can reverse the effects of CAFs and improve the responsiveness of PCa to chemotherapy.Conclusions
This study uncovers a paracrine mechanism of chemoresistance in PCa and proposes that targeting the stroma could be a therapeutic choice.
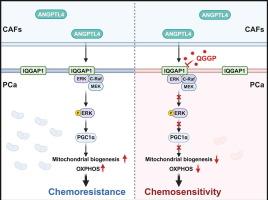
前列腺癌相关成纤维细胞通过ANGPTL4-IQGAP1轴调控线粒体代谢并抑制化疗敏感性
癌症相关成纤维细胞(CAFs)是肿瘤微环境的关键组成部分,与促进肿瘤生长和培养耐药性有关。尽管如此,它们在前列腺癌(PCa)中的作用机制仍不完全清楚,这对于制定有效的治疗策略至关重要。本研究的主要目的是探讨CAFs介导PCa生长和化疗耐药的机制。方法通过数据分析和实验验证了CAFs对前列腺癌细胞增殖和化疗耐药的影响。随后,我们对来自CAFs和PCa细胞的条件培养基进行了全面的蛋白质组学分析,并确定血管生成素样蛋白4 (ANGPTL4)是一个关键因素。ELISA和多重免疫荧光检测均表明,ANGPTL4主要由caf分泌。接下来,我们通过代谢组学分析、GST pull-down实验、Co-IP等实验,探索ANGPTL4的具体分子机制及其对PCa细胞的确切作用。通过药物筛选,我们鉴定出槲皮素3-O-(6′-半乳糖吡喃基)-β- d -半乳糖吡喃苷(QGGP)是一种有效的CAFs功能抑制剂。最后,我们全面评估了QGGP作为单一疗法和与多西紫杉醇联合治疗PCa细胞的治疗潜力。结果我们发现外分泌因子ANGPTL4主要在PCa的CAFs中表达。当ANGPTL4与PCa细胞膜上含有IQ基序的gtpase激活蛋白1 (IQGAP1)结合时,激活Raf-MEK-ERK-PGC1α轴,促进线粒体生物发生和OXPHOS代谢,从而促进PCa生长和耐药。此外,虚拟和功能筛选策略确定QGGP是促进其降解的IQGAP1的特异性抑制剂。QGGP联合多西紫杉醇治疗可逆转CAFs的作用,提高PCa对化疗的反应性。结论本研究揭示了前列腺癌化疗耐药的旁分泌机制,并提出靶向间质可能是一种治疗选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


