Analysis of spider silk in loading-unloading cycles using Raman spectroscopy based on molecular bioinformatics of spidrion
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
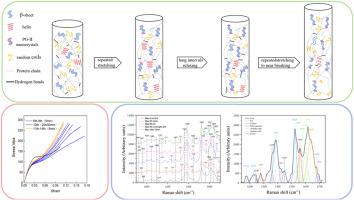
The mechanical properties of spider silk result from its organization at various levels, including the amino acid sequence, protein structure, protein assembly and full-hierarchical microstructure. However, the relatively few reports that contain an analysis of the motifs along the full-length of the sequences, and of the evolution of their secondary structure when the fiber is subjected to mechanical load, render difficult the task of relating sequence, microstructure and properties for this material. In this study, we identified seven spider major ampullate gland silk proteins of Argiope bruennichi, determine their full-length amino acid motifs, simulated the repeated stretching of spider major ampullate gland silk (MAS) in the natural environment, verified the stability of its mechanical properties, and established the evolution of its protein structure by semi-quantitative analysis of Raman spectroscopy. After stretching at different strains, MAS can recover previous mechanical behavior and exhibit excellent shape and mechanical memory in terms of longitudinal stretching. It is also shown that MAS maintain its mechanical properties through a precise adjustment of protein structure, secondary structure transformation and reconstruction. This study shows that the repeated stretching characteristics of spider major ampullate gland silk may be a post-processing adjustment way that, in combination with its molecular organization, may bring a new inspiration for the relation of sequence-structure-property.

基于蜘蛛粒子分子生物信息学的拉曼光谱分析蜘蛛丝的装卸循环
蜘蛛丝的力学性能是由其氨基酸序列、蛋白质结构、蛋白质组装和全层次结构等不同层次的组织结构决定的。然而,相对较少的报道包含沿序列全长的基序分析,以及当纤维受到机械载荷时其二级结构的演变,使得该材料的序列,微观结构和性能的相关任务变得困难。本研究鉴定了7种Argiope bruennichi蜘蛛壶腹腺丝蛋白,确定了它们的全长氨基酸基序,模拟了蜘蛛壶腹腺丝(spider major ampullate gland silk, MAS)在自然环境下的反复拉伸,验证了其力学性能的稳定性,并通过拉曼光谱半定量分析确定了其蛋白结构的演化。在不同应变下拉伸后,MAS可以恢复之前的力学行为,并在纵向拉伸方面表现出优异的力学记忆。研究还表明,MAS通过对蛋白质结构的精确调整、二级结构的转变和重建来维持其力学性能。本研究表明,蜘蛛主壶腹腺丝的重复拉伸特性可能是一种后处理调整方式,结合其分子组织结构,可能为序列-结构-性能关系带来新的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


