Reverse-engineered Electro-Fenton for the selective synthesis of oxalic or oxamic acid through the degradation of acetaminophen: a novel green electrocatalytic refinery approach
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
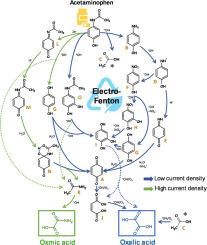
The Electro-Fenton process (EF) has been conventionally applied to efficiently degrade refractory and/or toxic pollutants. However, in this work, EF was used as a reverse engineering tool to selectively synthesize highly value-added products (oxalic or oxamic acid) through the degradation of the model pollutant acetaminophen, a widely used analgesic and antipyretic drug. It was found that the production of either oxalic or oxamic acid is dictated by the applied current density. Hence, oxalic acid is favored at low current densities trough a mechanism dominated by homogeneous •OH radical oxidation, while oxamic acid is the majoritarian product at high current densities where electron transfer at the anode surface becomes an important mechanism in combination with •OH oxidation. Under optimal reaction conditions (0.71 mA cm-2 and 100 mg L-1 of initial total organic carbon (TOC) concentration), up to 227.1 ± 26.3 mg L-1 of oxalic acid were produced, with high yield and selectivity of 54.9 ± 5.1% and 94.7 ± 9.9%, respectively (the TOC removal was 42.0 ± 2.4%). In the case of oxamic acid, the highest concentration of 33.8 ± 2.1 mg L-1 was produced at 2.13 mA cm-2 and an initial TOC concentration of 50 mg L-1, which represented a yield of 18.7 ± 0.3% and 60.9 ± 9.3% selectivity (71.1 ± 4.4% of TOC removal). It is worth noting that at low current density when oxalic acid is favored, the selectivity for both products was 100%, meaning that those were the only products remaining in the solution, with oxalic acid as the major product (94.7 ± 9.9% with initial TOC of 100 mg L-1, and 98.7 ± 0.9% with initial TOC of 50 mg L-1). This is a pioneer work on EF applications to the field of wastewater valorization/refining through the recovery of value-added products within a circular economy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


