Fabrication of lactoferrin-chitosan-etoposide nanoparticles with melatonin via carbodiimide coupling: In-vitro & in-vivo evaluation for colon cancer
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This study presents the development of melatonin-coated lactoferrin-chitosan nanoparticles (ETP-CS-LF-MLT-NPs) using ionic gelation and carbodiimide coupling for colorectal cancer treatment. The nanoparticles were characterized by an average size of 208.7 ± 1.25 nm, a zeta potential of 30.77 ± 1.21 mV, and 82.45 % drug encapsulation efficiency. In vitro drug release studies showed sustained, pH-responsive release, with 98.68 ± 4.12 % released at pH 5.5 over 24 h. The nanoparticles exhibited significant cytotoxicity in HCT116 cells (IC50 = 15.32 μg/mL), inducing ROS generation, apoptosis, and G2/M cell cycle arrest, with notable downregulation of BCL2 gene expression. Enhanced cellular uptake due to lactoferrin targeting improved therapeutic efficacy. In In vivo studies, the nanoparticles demonstrated significant tumor reduction and selective colon accumulation in a DMH-induced colorectal cancer rat model, along with improved pharmacokinetics, showing extended plasma circulation and bioavailability compared to free etoposide. Biocompatibility assays, including hemolysis (<1 %), platelet aggregation, and HET-CAM tests, confirmed the safety profiling of the prepared nanoparticles. The nanoparticles also inhibited Proteus mirabilis (ZOI = 1.9 cm) and exhibited promising effects on the gut microbiome of treated animals. Altogether, ETP-CS-LF-MLT-NPs hold great potential for targeted colorectal cancer therapy, improving drug delivery, tumor targeting, bioavailability, and reducing systemic toxicity.
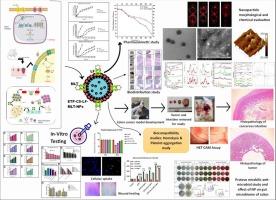
通过碳二亚胺偶联制备乳铁蛋白-壳聚糖-依托泊苷纳米颗粒与褪黑素:结肠癌的体外和体内评估。
本研究利用离子凝胶和碳二亚胺偶联制备褪黑素包被乳铁蛋白-壳聚糖纳米颗粒(ETP-CS-LF-MLT-NPs)用于结直肠癌治疗。纳米颗粒的平均粒径为208.7 ± 1.25 nm, zeta电位为30.77 ± 1.21 mV,包封率为82.45 %。体外释药研究显示,在pH 5.5下,24 h内释药率为98.68 ± 4.12 %。纳米颗粒在HCT116细胞中表现出明显的细胞毒性(IC50 = 15.32 μg/mL),诱导ROS生成、细胞凋亡和G2/M细胞周期阻滞,并显著下调BCL2基因表达。增强细胞摄取由于乳铁蛋白靶向提高治疗效果。在体内研究中,纳米颗粒在dmh诱导的结直肠癌大鼠模型中显示出显著的肿瘤减少和选择性结肠积聚,同时与游离依托泊苷相比,改善了药代动力学,显示出更长的血浆循环和生物利用度。生物相容性测定,包括溶血(
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


