Theoretical Insight into the Multiple Roles of the Silyl-Phenanthroline Ligand in Ir-Catalyzed C(sp3)–H Borylation
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
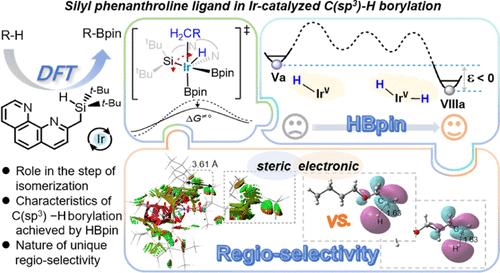
Silyl-phenanthroline (NN′Si) ligand ancillary iridium-catalyzed C(sp3)–H borylation is investigated theoretically. Density functional theory calculations clearly disclose that the (NN′Si)IrV(H)(Bpin)3 (NN′Si = 6-[(di-tert-butylsilyl)methyl]-1,10-phenanthroline) complex is a resting state, and the (NN′Si)IrIII(Bpin)2 complex serves as an active species in the catalytic cycle. The remarkably high activity of this type of a catalyst arises from the rapid reductive elimination of HBpin from (NN′Si)IrV(H)(Bpin)3 to generate the active species (NN′Si)IrIII(Bpin)2. The silyl group plays a crucial role in accelerating the crucial hydride-migration elementary step, which allows the isomerization of the (NN′Si)IrV(R)(H)(Bpin)2 intermediate to achieve the C(sp3)–B reductive elimination and afford the borylated product. Although C(sp3)–H borylation with HBpin is thermodynamically unfavorable, the Ir-dihydride intermediate (NN′Si)IrV(H)2(Bpin)2 generated after product formation is slightly more stable than resting-state (NN′Si)IrV(H)(Bpin)3 in this catalytic cycle, which is an important driving force for the HBpin reaction. Such success was not attained by many other traditional bidentate ligands. The unique regioselectivity of n-butyl ethyl ether and 2-methylheptane, induced by the NN′Si-pincer ligand, is well reproduced and the underlying reason for the selectivity is clearly elucidated.
硅基-菲罗啉配体在ir催化C(sp3) -H硼化反应中多重作用的理论认识
从理论上研究了硅基-菲罗啉(NN 'Si)配体辅助铱催化的C(sp3) -H硼化反应。密度泛函理论计算清楚地表明(NN 'Si)IrV(H)(Bpin)3 (NN 'Si = 6-[(二叔丁基硅基)甲基]-1,10-菲罗啉)配合物为静息态,(NN 'Si)IrIII(Bpin)2配合物在催化循环中为活性物质。这类催化剂的高活性源于HBpin在(NN’si)IrV(H)(Bpin)3中的快速还原消除,生成活性物质(NN’si)IrIII(Bpin)2。硅基在加速关键的氢化物迁移基本步骤中起着至关重要的作用,使(NN’si)IrV(R)(H)(Bpin)2中间体异构化,从而实现C(sp3) -B的还原消除,得到硼化产物。虽然C(sp3) -H与HBpin的硼化反应在热力学上是不利的,但在该催化循环中产物形成后生成的ir -二氢化物中间体(NN 'Si)IrV(H)2(Bpin)2比静息态(NN 'Si)IrV(H)(Bpin)3稍稳定,这是HBpin反应的重要驱动力。这种成功是许多其他传统双齿配体所不能达到的。正丁基乙醚和2-甲基庚烷在NN ' si -钳子配体诱导下的独特的区域选择性得到了很好的再现,并清楚地阐明了这种选择性的根本原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


