Achieving reversible Mg chemistry by tunning electric double layer structure with highly-fluorinated asymmetric magnesium salt
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
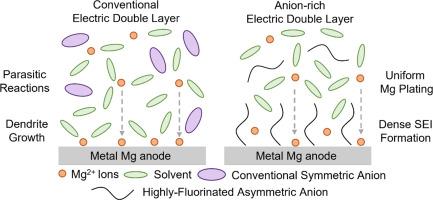
The interfacial instability of the Mg metal in chlorine-free magnesium electrolytes severely hinders the practical applications of rechargeable magnesium batteries (RMBs). Conventionally, the reversibility and longevity of Mg metal have been primarily associated with the bulk Mg2+ solvation structure in organic electrolytes. However, the electric double layer (EDL) is the real microscopic region where all electrochemical reactions occur. With the region, both electronic and ionic charges undergo periodic rearrangement with surface charge fluctuation during electrochemical processes. Consequently, the structure and properties of EDL critically influence the Mg electroplating process, which has been severely overlooked for a long time. Here, for the first time, we propose a novel strategy of tuning the anion structure in Mg salt to rearrange EDL, thereby simultaneously regulating the uniformity of electroplated Mg morphology and building an inorganic-rich, high-quality solid electrolyte interphase (SEI) layer, achieving a highly reversible Mg anode. We designed a novel highly fluorinated asymmetric Mg salt, magnesium perfluoro(2-ethoxyethane)sulfonate (Mg(PES)2), which endows the Mg metal anode with superior Coulombic efficiency (CE) of over 99.2%, ultra-low overpotential of 20 mV and remarkable cyclability of over 4500 h. This work introduces a new strategy for multivalent cation anodes, emphasizing interfacial chemistry regulation over traditional bulk solvation structure design. We believe this insightful approach will open up new frontiers in the design for advanced multivalent electrolyte development.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


