Co-occurrence of cadmium and ciprofloxacin in environmental media decreases ciprofloxacin degradation by biogenic manganese oxides
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The coexistence of antibiotics with heavy metals is detrimental to humans and the environment. In urban water environments, Cadmium (Cd) and ciprofloxacin (CIP) frequently co-occur. Biogenic manganese oxides (BMOs) are a promising environmental bioremediation material due to their remarkable adsorption and oxidation properties. However, BMOs' removal mechanism in an environment where Cd and CIP co-occur is not yet unknown. We identified a manganese (Mn)-oxidising bacterium, Bacillus sp. XM02, with a strong ability for Mn (II) oxidation (85.23%) and BMOs production, and investigated its competitive removal mechanism in an environment with Cd and CIP co-occurrence. The BMOs exhibited a glorious CIP degradation ability and led to a marked decrease in the toxicity of CIP following oxidative degradation in Escherichia coli experiments. In contrast, in the co-existence of Cd and CIP, Cd hindered CIP removal by BMOs, but CIP did not affect Cd removal. Kinetic experiments combined with XPS characterisation revealed that the k value of Cd (297.39 h−1) was much higher than that of CIP (5.53 h−1), demonstrating that Cd was immediately adsorbed onto the surface of BMOs through a Cd-O bond. The surface potentials of BMOs carrying Cd alone and both Cd and CIP on the surface were similar, revealing that the electronegativity of Cd-carrying BMOs was greatly weakened (from −34.8 mV to −21 mV/-23 mV), which further reduced the BMOs' electrostatic interaction with CIP. Moreover, the concentration of dissolved Mn (III) in the co-existence group was lower than that in the CIP alone, indicating that the presence of Cd reduced the transformation of Mn (IV) to Mn (III) by BMOs. Consequently, Cd attenuated the effect of active Mn (IV) sites of BMOs on CIP's piperazine ring oxidative degradation. These results offer a theoretical direction for the use of BMOs to reduce the risk posed by antibiotics and heavy metals pollution in co-occurrence environments.
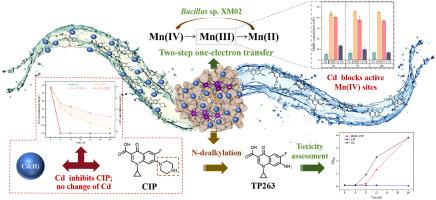

环境介质中镉和环丙沙星的共存降低了生物源性锰氧化物对环丙沙星的降解
抗生素与重金属共存对人类和环境都是有害的。在城市水环境中,镉(Cd)和环丙沙星(CIP)经常共存。生物源性锰氧化物(BMOs)具有良好的吸附和氧化性能,是一种很有前途的环境生物修复材料。然而,在Cd和CIP共存的环境中,BMOs的去除机制尚不清楚。本研究鉴定了一株锰(Mn)氧化菌芽孢杆菌(Bacillus sp. XM02),该细菌具有较强的Mn (II)氧化能力(85.23%)和BMOs生成能力,并研究了其在Cd和CIP共存环境下的竞争去除机制。在大肠杆菌实验中,BMOs表现出良好的CIP降解能力,并导致CIP氧化降解后的毒性显著降低。而在Cd和CIP共存的情况下,Cd阻碍了BMOs对CIP的去除,而CIP对Cd的去除没有影响。动力学实验结合XPS表征表明,Cd的k值(297.39 h-1)远高于CIP的k值(5.53 h-1),表明Cd通过Cd- o键直接吸附在BMOs表面。单独携带Cd的BMOs和同时携带Cd和CIP的BMOs表面电位相似,表明携带Cd的BMOs的电负性大大减弱(从-34.8 mV降至-21 mV/-23 mV),这进一步降低了BMOs与CIP的静电相互作用。此外,共存组中溶解的Mn (III)浓度低于单独CIP组,表明Cd的存在减少了BMOs对Mn (IV)向Mn (III)的转化。因此,Cd减弱了BMOs的活性Mn (IV)位点对CIP的哌嗪环氧化降解的影响。这些结果为在共生环境中使用BMOs降低抗生素和重金属污染的风险提供了理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


