Light-Driven Hybrid Nanoreactor Harnessing the Synergy of Carboxysomes and Organic Frameworks for Efficient Hydrogen Production
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Synthetic photobiocatalysts are promising catalysts for valuable chemical transformations by harnessing solar energy inspired by natural photosynthesis. However, the synergistic integration of all of the components for efficient light harvesting, cascade electron transfer, and efficient biocatalytic reactions presents a formidable challenge. In particular, replicating intricate multiscale hierarchical assembly and functional segregation involved in natural photosystems, such as photosystems I and II, remains particularly demanding within artificial structures. Here, we report the bottom-up construction of a visible-light-driven chemical–biological hybrid nanoreactor with augmented photocatalytic efficiency by anchoring an α-carboxysome shell encasing [FeFe]-hydrogenases (H–S) on the surface of a hydrogen-bonded organic molecular crystal, a microporous α-polymorph of 1,3,6,8-tetra(4′-carboxyphenyl)pyrene (TBAP-α). The self-association of this chemical–biological hybrid system is facilitated by hydrogen bonds, as revealed by molecular dynamics simulations. Within this hybrid photobiocatalyst, TBAP-α functions as an antenna for visible-light absorption and exciton generation, supplying electrons for sacrificial hydrogen production by H–S in aqueous solutions. This coordination allows the hybrid nanoreactor, H–S|TBAP-α, to execute hydrogen evolution exclusively driven by light irradiation with a rate comparable to that of photocatalyst-loaded precious cocatalyst. The established approach to constructing new light-driven biocatalysts combines the synergistic power of biological nanotechnology with the multilength-scale structure and functional control offered by supramolecular organic semiconductors. It opens up innovative opportunities for the fabrication of biomimetic nanoreactors for sustainable fuel production and enzymatic reactions.
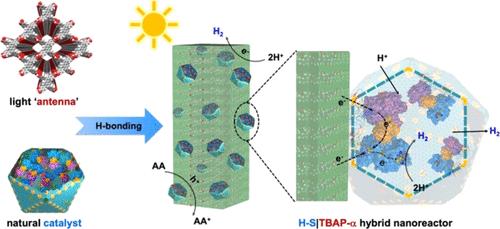
利用羧基体和有机框架协同作用的光驱动混合纳米反应器高效制氢
合成光生物催化剂是一种很有前途的催化剂,通过利用自然光合作用激发的太阳能来进行有价值的化学转化。然而,高效光收集、级联电子转移和高效生物催化反应的所有组件的协同集成提出了一个艰巨的挑战。特别是,在人工结构中,复制自然光系统(如光系统I和II)中涉及的复杂的多尺度分层组装和功能分离仍然是特别需要的。在这里,我们报道了一个由下向上构建的可见光驱动化学-生物杂化纳米反应器,通过在氢键有机分子晶体(1,3,6,8-四(4 ' -羧基苯基)芘(TBAP-α)的微孔α-多晶)表面锚定α-羧基体外壳包裹[FeFe]-氢化酶(H-S),增强了光催化效率。分子动力学模拟表明,氢键促进了这种化学-生物杂化体系的自结合。在这种混合型光生物催化剂中,ttbap -α作为可见光吸收和激子产生的天线,为水溶液中H-S的牺牲产氢提供电子。这种配位使得混合纳米反应器H-S |TBAP-α能够完全由光照射驱动析氢,其速率与负载宝贵助催化剂的光催化剂相当。构建新型光驱动生物催化剂的既定方法将生物纳米技术的协同能力与超分子有机半导体提供的多长度尺度结构和功能控制相结合。它为制造用于可持续燃料生产和酶促反应的仿生纳米反应器开辟了创新机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


