Chemical Torque in Y14Ag39.3Zn12.1: Unwinding the Disordered Triangles of the Gd14Ag51 Type
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
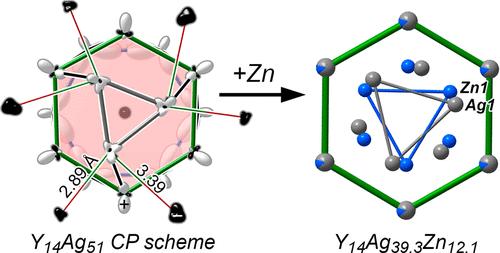
Elemental substitution is commonly used in materials chemistry to tune the Fermi energy of a compound or impart Chemical Pressure (CP) to atomic environments. In this Article, we illustrate how such substitution can also allow one to tune the relative orientations of geometrical units within a crystal structure, using the Gd14Ag51 structure type as a demonstration. A key feature of this structure type is the presence of hexagonal columns based on the CaPd5+x structure type, containing triangles of atoms disordered over two orientations. Synthesis and structure solution of a Zn-substituted variant of this structure, Y14Ag39.3Zn12.1, reveal that the incorporation of Zn atoms into these triangles leads to their rotation relative to their surroundings. Distinct triangle orientations are found depending on whether they are Ag3 or Zn3 units. A DFT-CP analysis of unsubstituted parent compound Y14Ag51 elucidates these observations. The Ag3 triangles lie within a large hexagon of Y atoms, with each triangle corner being able to gain close contacts with up to two of these Y atoms depending on the orientation. Obtaining an optimal alignment of the Ag3 triangles with respect to these interactions, however, is prevented by repulsion from other Ag atoms in the columns derived from the CaPd5+x type. Instead, the triangles are twisted toward the Y neighbors with larger negative CP features. The replacement of Ag atoms with smaller Zn atoms provides the opportunity to relieve these packing tensions, allowing the triangles to turn to a position that better optimizes their interactions with the surrounding Y atoms. These results point to simple guidelines for identifying, with CP analysis, rigid units within structures that may be manipulated through elemental substitution.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


