Targeted Delivery of siRNA-Gemcitabine Oligonucleotide Chimeras for High-Efficacy Synergistic Treatment of Pancreatic Cancer
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
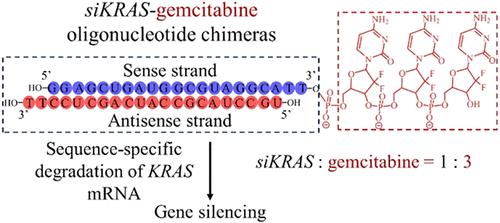
Pancreatic cancer (PC) stands for the most intractable malignancy. Gemcitabine (GEM) is one of the few approved first-line treatments for PC patients. The fast clearance, demanded high dosage, and existence of drug resistance have, nevertheless, posed not only a significant limitation to its clinical efficacy but also serious toxicity concerns. KRASG12D mutation is identified as a key driver in many PC patients, and its expression level shows a correlation with drug resistance and mortality. Here, we explored KRASG12D siRNA-gemcitabine oligonucleotide chimeras (siKRAS-Gn) as a dual prodrug that was designed to specifically silence KRASG12D gene and sensitize PC cells to GEM for the synergistic treatment of PC. siKRAS-Gn conjugates with 1, 2, 3, 4, or 5 units of GEM were synthesized and delivered using cRGD-decorated polymersomes. Interestingly, the proapoptotic activity of siKRAS-Gn was shown to highly depend on the number of GEM, in which three GEM units (siKRAS-G3) were found to be optimal and induced strong apoptosis of PANC-1 cells (apoptosis rate: 64.2%). In contrast, minimal cell apoptosis was discerned for siKRAS, siKRAS-G1, siKRAS-G2, and free GEM (9-fold of that in siKRAS-G3). siKRAS-G3, while showing similar KRAS mRNA silencing ability to siKRAS, markedly enhanced the downregulation of KRAS protein in vitro and in vivo. Accordingly, siKRAS-G3 significantly outperformed siKRAS and siScramble-G3 in both tumor inhibition and survival benefits. The targeted delivery of the siKRAS-gemcitabine prodrug conjugate has emerged as an appealing treatment for pancreatic cancer.
靶向递送sirna -吉西他滨寡核苷酸嵌合体高效协同治疗胰腺癌
胰腺癌(PC)是最难治性的恶性肿瘤。吉西他滨(GEM)是为数不多的批准用于PC患者的一线治疗药物之一。但其清除率快、剂量大、耐药等特点不仅严重限制了其临床疗效,而且存在严重的毒性问题。KRASG12D突变是许多PC患者的关键驱动因素,其表达水平与耐药和死亡率相关。在这里,我们探索KRASG12D sirna -吉西他滨寡核苷酸嵌合体(siKRAS-Gn)作为双前药,旨在特异性沉默KRASG12D基因并使PC细胞对GEM敏感,以协同治疗PC。siKRAS-Gn与1、2、3、4或5个GEM的偶联物被合成并使用crgd修饰的聚合体递送。有趣的是,siKRAS-Gn的促凋亡活性高度依赖于GEM的数量,其中3个GEM单位(siKRAS-G3)表现最佳,诱导PANC-1细胞强烈凋亡(凋亡率:64.2%)。相比之下,siKRAS、siKRAS- g1、siKRAS- g2和游离GEM的细胞凋亡最小(是siKRAS- g3的9倍)。siKRAS- g3在表现出与siKRAS相似的KRAS mRNA沉默能力的同时,在体外和体内均显著增强了KRAS蛋白的下调。因此,siKRAS- g3在肿瘤抑制和生存获益方面明显优于siKRAS和siScramble-G3。靶向递送sikras -吉西他滨前药偶联物已成为胰腺癌的一种有吸引力的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


