Using Data-Science Approaches to Unravel Insights for Enhanced Transport of Lithium Ions in Single-Ion Conducting Polymer Electrolytes
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
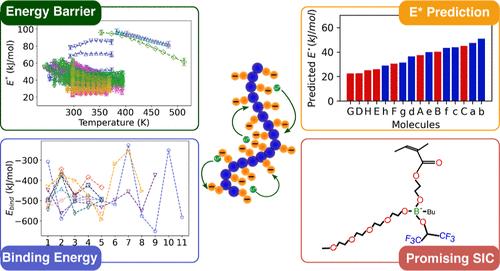
Solid polymer electrolytes have yet to achieve the desired ionic conductivity (>1 mS/cm) near room temperature required for many applications. This target implies the need to reduce the effective energy barriers for ion transport in polymer electrolytes to around 20 kJ/mol. In this work, we combine information extracted from existing experimental results with theoretical calculations to provide insights into ion transport in single-ion conductors (SICs) with a focus on lithium ion SICs. Through the analysis of temperature-dependent ionic conductivity data obtained from the literature, we evaluate different methods of extracting energy barriers for lithium transport. The traditional Arrhenius fit to the temperature-dependent ionic conductivity data indicates that the Meyer–Neldel rule holds for SICs. However, the values of the fitting parameters remain unphysical. Our modified approach based on recent work (Macromolecules 2023, 56, 15, 6051), which incorporates a fixed pre-exponential factor, reveals that the energy barriers exhibit temperature dependence over a wide range of temperatures. Using this approach, we identify anions leading to the energy barriers <30 kJ/mol, which include trifluoromethane sulfonimide (TFSI), fluoromethane sulfonimide (FSI), and boron-based organic anions. In our efforts to design the next generation of anions, which can exhibit the energy barriers <20 kJ/mol, we have performed density functional theory (DFT) based calculations to connect the chemical structures of boron-based anions via the binding energy of cation (lithium)-anion pairs with the experimentally derived effective energy barriers for ion hopping. Not only have we identified a correlation between the binding energy and the energy barriers, but we also propose a strategy to design new boron-based anions by using the correlation. This combined approach involving experiments and theoretical calculations is capable of facilitating the identification of promising new anions, which can exhibit ionic conductivity >1 mS/cm near room temperature, thereby expediting the development of novel superionic single-ion conducting polymer electrolytes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


