Nonheme iron catalyst mimics heme-dependent haloperoxidase for efficient bromination and oxidation
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The [Fe]/H2O2 oxidation system has found wide applications in chemistry and biology. Halogenation with this [Fe]/H2O2 oxidation protocol and halide (X−) in the biological system is well established with the identification of heme-iron–dependent haloperoxidases. However, mimicking such halogenation process is rarely explored for practical use in organic synthesis. Here, we report the development of a nonheme iron catalyst that mimics the heme-iron–dependent haloperoxidases to catalyze the generation of HOBr from H2O2/Br− with high efficiency. We discovered that a tridentate terpyridine (TPY) ligand designed for Fenton chemistry was optimal for FeBr3 to form a stable nonheme iron catalyst [Fe(TPY)Br3], which catalyzed arene bromination, Hunsdiecker-type decarboxylative bromination, bromolactonization, and oxidation of sulfides and thiols. Mechanistic studies revealed that Fenton chemistry ([Fe]/H2O2) might operate to generate hydroxyl radical (HO•), which oxidize bromide ion [Br−] into reactive HOBr. This nonheme iron catalyst represents a biomimetic model for heme-iron–dependent haloperoxidases with potential applications in organic synthesis, drug discovery, and biology.
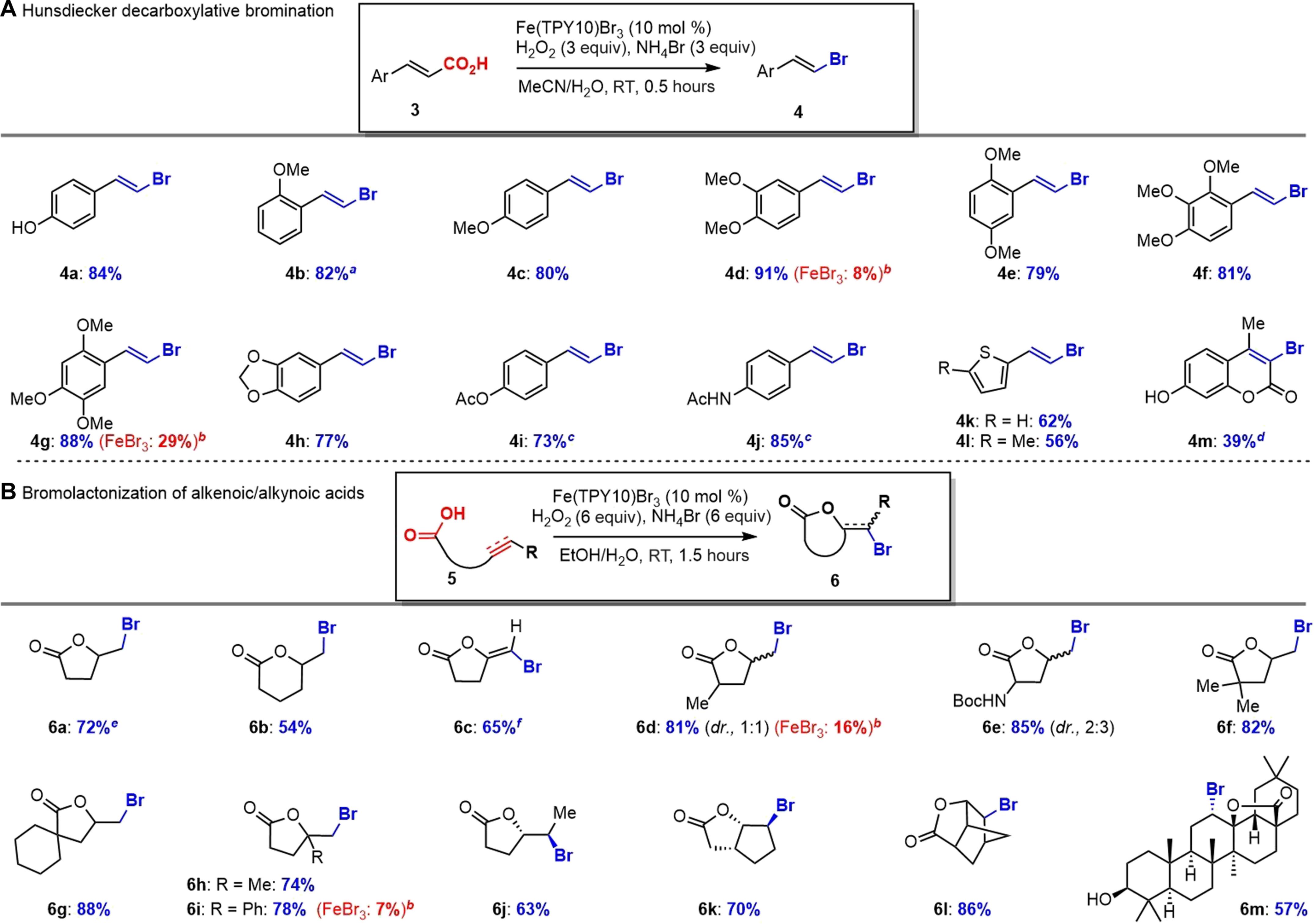
非血红素铁催化剂模拟血红素依赖的卤素过氧化物酶进行有效的溴化和氧化。
[Fe]/H2O2氧化体系在化学和生物学中有着广泛的应用。这种[Fe]/H2O2氧化方案和卤化物(X-)在生物系统中的卤化已经很好地建立了血红素-铁依赖性卤化过氧化物酶的鉴定。然而,模拟这种卤化过程很少探索在有机合成中的实际应用。在这里,我们报道了一种非血红素铁催化剂的开发,该催化剂模拟血红素铁依赖性卤素过氧化物酶,以高效催化H2O2/Br-生成HOBr。我们发现,为Fenton化学设计的三齿三联吡啶(TPY)配体最适合于fe2o3形成稳定的非血红素铁催化剂[Fe(TPY)Br3],催化芳烃溴化、hunsdiecker型脱羧溴化、溴丙酮化以及硫化物和硫醇的氧化。机理研究表明,Fenton化学([Fe]/H2O2)可能产生羟基自由基(HO•),将溴离子[Br-]氧化为反应性HOBr。这种非血红素铁催化剂代表了血红素铁依赖性卤素过氧化物酶的仿生模型,在有机合成、药物发现和生物学方面具有潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


