A trafficking regulatory subnetwork governs αVβ6 integrin-HER2 cross-talk to control breast cancer invasion and drug resistance
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
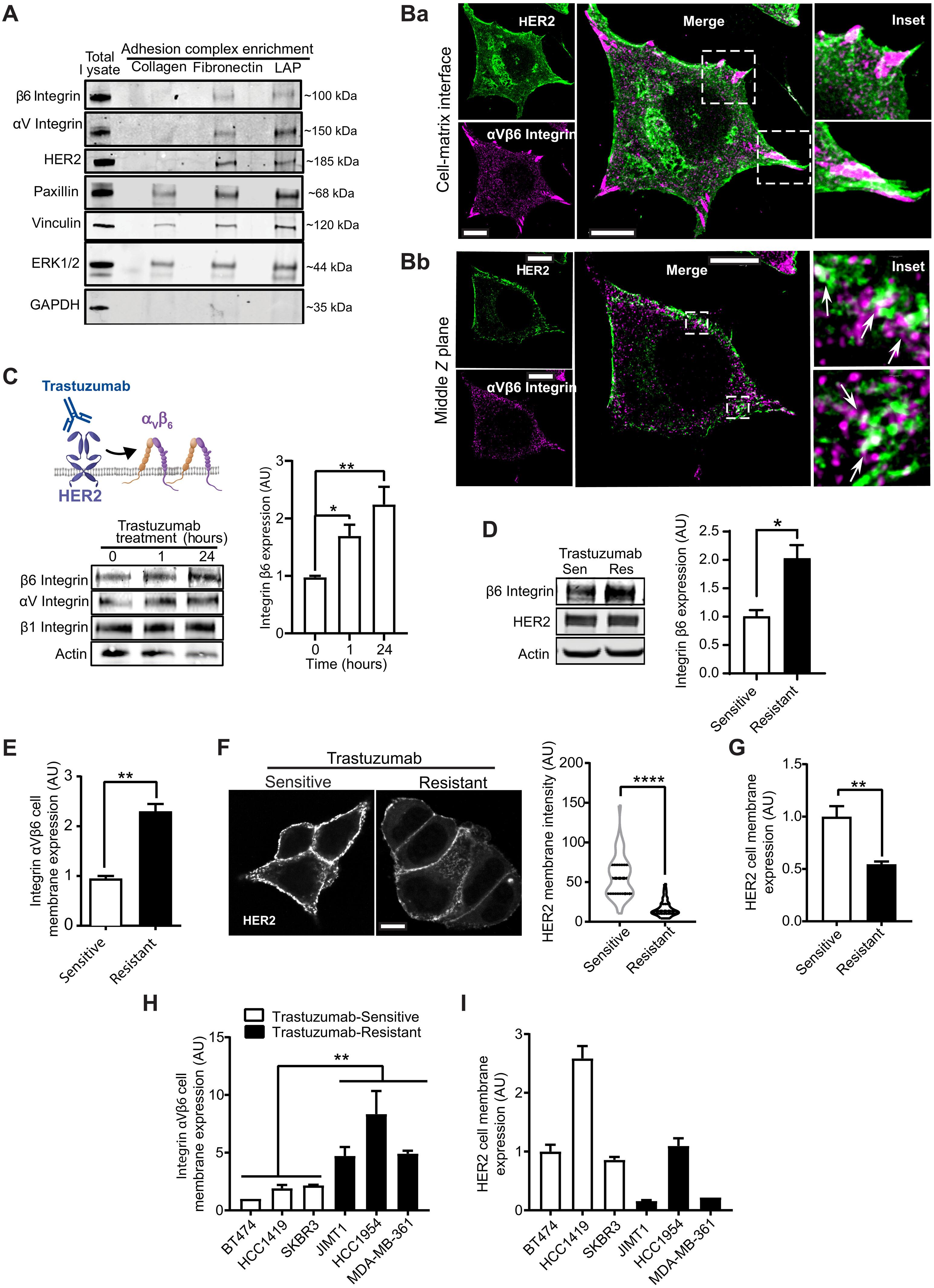
HER2 and αVβ6 integrin are independent predictors of breast cancer survival and metastasis. We identify an αVβ6/HER2 cross-talk mechanism driving invasion, which is dysregulated in drug-resistant HER2+ breast cancer cells. Proteomic analyses reveal ligand-bound αVβ6 recruits HER2 and a trafficking subnetwork, comprising guanosine triphosphatases RAB5 and RAB7A and the Rab regulator guanine nucleotide dissociation inhibitor 2 (GDI2). The RAB5/RAB7A/GDI2 functional module mediates direct cross-talk between αVβ6 and HER2, affecting receptor trafficking and signaling. Acute exposure to trastuzumab increases recruitment of the subnetwork to αVβ6, but trastuzumab resistance decouples GDI2 recruitment. GDI2, RAB5, and RAB7A cooperate to regulate migration and transforming growth factor–β activation to promote invasion. However, these mechanisms are dysregulated in trastuzumab-resistant cells. In patients, RAB5A, RAB7A, and GDI2 expression correlates with patient survival and αVβ6 expression predicts relapse following trastuzumab treatment. Thus, the RAB5/RAB7A/GDI2 subnetwork regulates αVβ6-HER2 cross-talk to drive breast cancer invasion but is subverted in trastuzumab-resistant cells to drive αVβ6-independent and HER2-independent tumor progression.
α v - β6整合素- her2串扰调控乳腺癌侵袭及耐药
HER2和αVβ6整合素是乳腺癌生存和转移的独立预测因子。我们发现αVβ6/HER2串扰机制驱动侵袭,该机制在耐药HER2+乳腺癌细胞中失调。蛋白质组学分析显示,配体结合的αVβ6招募HER2和一个转运亚网络,包括鸟苷三磷酸酶RAB5和RAB7A以及鸟苷调节因子鸟嘌呤核苷酸解离抑制剂2 (GDI2)。RAB5/RAB7A/GDI2功能模块介导αVβ6与HER2之间的直接串扰,影响受体转运和信号转导。急性暴露于曲妥珠单抗会增加αVβ6的子网络募集,但曲妥珠单抗耐药性会使GDI2募集解耦。GDI2、RAB5和RAB7A协同调节迁移和转化生长因子-β活化,促进侵袭。然而,这些机制在曲妥珠单抗耐药细胞中失调。在患者中,RAB5A、RAB7A和GDI2表达与患者生存相关,αVβ6表达预测曲妥珠单抗治疗后复发。因此,RAB5/RAB7A/GDI2子网络调节αVβ6-HER2串扰驱动乳腺癌侵袭,但在曲珠单抗耐药细胞中被破坏,驱动α v β6非依赖性和her2非依赖性肿瘤进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


