Additives Capable of Stably Supplying Anions/Cations for Homogeneous Lithium Deposition/Stripping
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
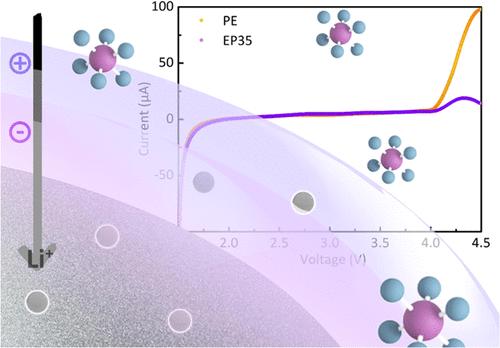
One of the important factors leading to lithium dendrites is a slow lithium-ion mass transport and imbalanced distribution of the Li+ concentration and nuclei sites on the anode surface. To achieve uniform lithium deposition during the charge and discharge process, we introduce a homemade new copolymer (with the quaternary ammonium group N3R+I– on its side chain as the main functional group), named P35, as a functional electrolyte additive to regulate the lithium deposition. Theoretical calculations show that under the strong coordinating interaction between I– and N3R+, P35 preferentially adsorbs onto the lithium foil surface, effectively countering the adsorption of lithium salt anions such as PF6–. Moreover, the positive charge carried by the quaternary ammonium salt group of P35 could interact with PF6– to limit their mobility. Consequently, the dipole interaction on lithium ions is diminished, leading to an enhancement in the transport rate and a decrease in the concentration gradient of lithium ions. Furthermore, a more efficient SEI was formed due to the dual charges electrostatic shield formed by N3R+I–. Li–Li symmetric cells and Li–LiFePO4 full cells assembled with electrolytes with P35 exhibit better rate performance, smaller polarization, and smoother deposition morphology in comparison to the cells without the P35 additive.
能稳定供应阴离子/阳离子的添加剂,用于均匀锂沉积/剥离
导致锂枝晶形成的重要因素之一是锂离子质量输运缓慢、锂离子浓度和锂离子核位在阳极表面分布不平衡。为了在充放电过程中实现均匀的锂沉积,我们引入了一种自制的新型共聚物(其侧链上的季铵盐基团N3R+I -为主要官能团)P35作为功能电解质添加剂来调节锂的沉积。理论计算表明,在I -与N3R+的强配位相互作用下,P35优先吸附在锂箔表面,有效对抗了PF6 -等锂盐阴离子的吸附。此外,P35的季铵盐基团携带的正电荷可能与PF6 -相互作用,限制了它们的迁移。因此,锂离子上的偶极相互作用减弱,导致输运速率增强和锂离子浓度梯度降低。此外,由于N3R+I -形成的双电荷静电屏蔽,形成了更高效的SEI。与不添加P35的锂离子电池相比,添加P35的锂离子对称电池和锂lifepo4全电池表现出更好的倍率性能、更小的极化和更平滑的沉积形貌。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


