Core- versus End-Alkylation: Tailoring Solid-State Structures and Properties of Near-Infrared-Absorbing Organic Semiconductors Based on Naphthodithiophenediones
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
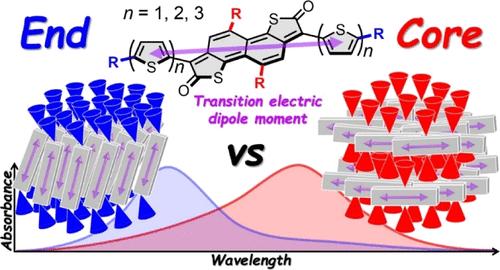
In the design of near-infrared (NIR)-absorbing organic semiconductors, not only chromophoric π-conjugated backbones that govern the molecular electronic structures but also solubilizing substituents, which can significantly affect the solid-state packing structures, are crucial factors in tailoring solid-state optical and electrical properties as well as solution processability. In this study, we systematically investigated the structures and properties of a series of core- and end-alkylated donor–acceptor–donor triads based on a naphthodithiophenedione acceptor to elucidate the impact of the alkylation site on their solid-state structures and properties. Although the alkylation site marginally affects their molecular electronic structures, all of the core-alkylated triads showed significant red shifts of the main absorption band from the solution to the solid state, in sharp contrast to the blue shifts observed for the end-alkylated triads. Single-crystal X-ray analysis and thin-film X-ray diffraction revealed that the contrasting solid-state optical properties are likely attributed to the distinctly different arrangements of the chromophores in the solid state, depending on the alkylation site. The end-alkylated triads tend to form interchromophore cofacial and/or side-by-side arrangements, which results in the blue shift of absorption bands, whereas the core-alkylated triads tend to avoid such interchromophore arrangement and rather promote an in-plane orientation of the transition electric dipole moments in a slip-stacking manner, which leads to the red shift of absorption bands. Furthermore, the alkyl groups on the core effectively reduce the core-to-core interaction, thus improving the solubility of materials without compromising carrier transport properties. These results provide valuable insights into molecular design for developing solution-processable NIR-absorbing organic semiconductors.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


