Discovery of ART0380, a Potent and Selective ATR Kinase Inhibitor Undergoing Phase 2 Clinical Studies for the Treatment of Advanced or Metastatic Solid Cancers
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
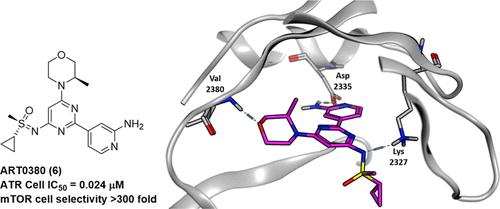
One of the hallmarks of cancer is high levels of DNA replication stress and defects in the DNA damage response (DDR) pathways, which are critical for maintaining genomic integrity. Ataxia telangiectasia and Rad3-related protein (ATR) is a key regulator of the DDR machinery and an attractive therapeutic target, with multiple ATR inhibitors holding significant promise in ongoing clinical studies. Herein, we describe the discovery and characterization of ART0380 (6), a potent and selective ATR inhibitor with a compelling in vitro and in vivo pharmacological profile currently undergoing Phase 2 clinical studies in patients with advanced or metastatic solid tumors as monotherapy and in combination with DNA-damaging agents (NCT04657068 and NCT05798611). ART0380 (6) has a favorable human PK profile suitable for both intermittent and continuous once-daily (QD) dosing, characterized by a dose-proportional increase in exposure and low variability.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


