Optimization and Biological Evaluation of Novel 1H-Pyrrolo[2,3-c]pyridin Derivatives as Potent and Reversible Lysine Specific Demethylase 1 Inhibitors for the Treatment of Acute Myelogenous Leukemia
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
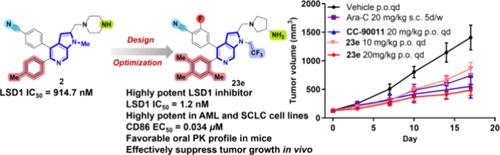
Lysine-specific demethylase 1 (LSD1) plays a vital role in the epigenetic regulation of various cancers, making it a promising therapeutic target for anticancer treatments. Herein, we designed and synthesized a novel series of 1H-pyrrolo[2,3-c]pyridin derivatives as potent LSD1 inhibitors. A detailed structure–activity relationship exploration was carried out to discover multiple derivatives with nanomolar enzymatic IC50 values. Further biological evaluation demonstrated that these compounds acted as selective and reversible LSD1 inhibitors. The representative compounds exhibited highly potent antiproliferative activity against both AML (MV4–11 and Kasumi-1) and SCLC (NCI-H526) cell lines. Additionally, they effectively activated CD86 mRNA expression in MV4–11 cells and induced differentiation of AML cell lines. Notably, the most promising compound 23e showed a favorable oral PK profile and effectively suppressed the tumor growth in an AML xenograft model. Overall, our medicinal chemistry efforts provide compound 23e as a lead compound for developing LSD1 inhibitors for the treatment of AML and other advanced malignancies.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


