TBD-catalyzed anionic ring-opening polymerization of hexamethylcyclotrisiloxane: a new route for the controlled synthesis of PDMS†
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
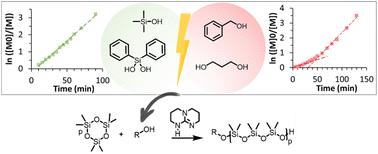
The controlled anionic ring-opening polymerization of hexamethylcyclotrisiloxane (D3) was optimized for a commercial and easy-to-use organic catalyst, 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), employing various types of initiators. Alcohols and silanols were compared for the synthesis of monofunctional and telechelic PDMS. Polymerizations were monitored by 1H NMR spectroscopy, MALDI-TOF mass spectroscopy and SEC to compare the effects of the initiator structure and catalyst concentration. TBD was shown to control the AROP of D3 when using silanols as the initiator, thus affording well-defined PDMS structures. When alcohols were used as initiators, the polymerization proceeded with a lower level of control due to slower initiation. These differences in initiation rates likely originated from the differences in pKa between silanols and alcohols.
tbd催化阴离子开环聚合六甲基环三硅氧烷:一种可控合成PDMS的新途径
采用多种引发剂对六甲基环三硅氧烷(D3)的可控阴离子开环聚合进行了优化,得到了易于使用的商业化有机催化剂1,5,7-三氮杂环[4.4.0]十二-5-烯(TBD)。比较了醇类和硅烷醇类合成单官能团和远旋团PDMS的方法。采用1H NMR谱、MALDI-TOF质谱和SEC对聚合过程进行了监测,比较了引发剂结构和催化剂浓度对聚合过程的影响。当使用硅烷醇作为引发剂时,TBD可以控制D3的AROP,从而提供定义明确的PDMS结构。当使用醇作为引发剂时,由于引发速度较慢,聚合过程的控制水平较低。这些起始速率的差异可能源于硅烷醇和醇之间pKa的差异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


