RANK drives structured intestinal epithelial expansion during pregnancy
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
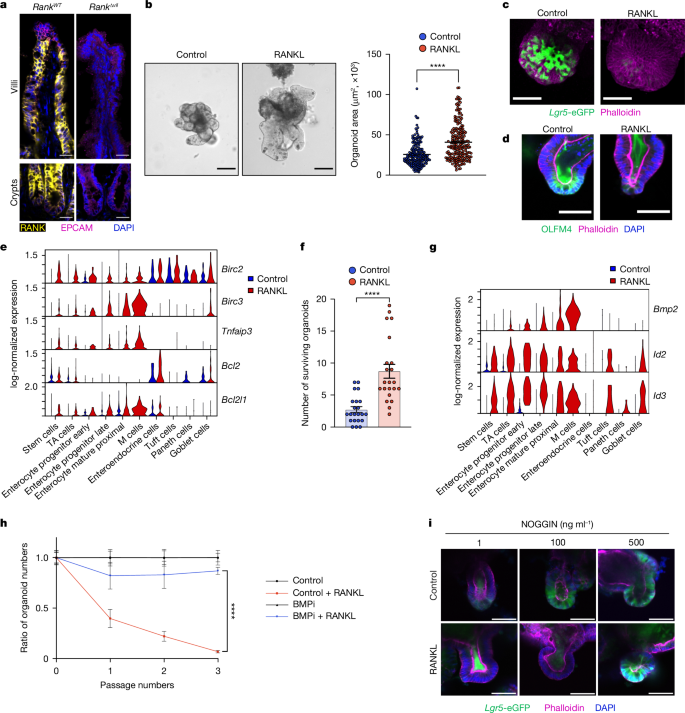
During reproduction, multiple species such as insects and all mammals undergo extensive physiological and morphological adaptions to ensure health and survival of the mother and optimal development of the offspring. Here we report that the intestinal epithelium undergoes expansion during pregnancy and lactation in mammals. This enlargement of the intestinal surface area results in a novel geometry of expanded villi. Receptor activator of nuclear factor-κΒ (RANK, encoded by TNFRSF11A) and its ligand RANKL were identified as a molecular pathway involved in this villous expansion of the small intestine in vivo in mice and in intestinal mouse and human organoids. Mechanistically, RANK–RANKL protects gut epithelial cells from cell death and controls the intestinal stem cell niche through BMP receptor signalling, resulting in the elongation of villi and a prominent increase in the intestinal surface. As a transgenerational consequence, babies born to female mice that lack Rank in the intestinal epithelium show reduced weight and develop glucose intolerance after metabolic stress. Whereas gut epithelial remodelling in pregnancy/lactation is reversible, constitutive expression of an active form of RANK is sufficient to drive intestinal expansion followed by loss of villi and stem cells, and prevents the formation of Apcmin-driven small intestinal stem cell tumours. These data identify RANK–RANKL as a pathway that drives intestinal epithelial expansion in pregnancy/lactation, one of the most elusive and fundamental tissue remodelling events in mammalian life history and evolution. The RANK–RANKL pathway drives intestinal epithelial expansion in pregnancy and lactation.

RANK驱动妊娠期肠道上皮结构扩张
在繁殖过程中,许多物种(如昆虫和所有哺乳动物)都经历了广泛的生理和形态适应,以确保母亲的健康和生存以及后代的最佳发育。在这里,我们报道了哺乳动物在怀孕和哺乳期间肠上皮的扩张。这种肠表面积的扩大导致肠绒毛扩张的新几何形状。核因子受体激活因子-κΒ (Receptor activator of nuclear factor-κΒ, RANK,由TNFRSF11A编码)及其配体RANKL在小鼠体内、肠道小鼠和人类器官中被鉴定为参与小肠绒毛扩张的分子途径。在机制上,RANK-RANKL保护肠道上皮细胞免于细胞死亡,并通过BMP受体信号传导控制肠道干细胞生态位,导致绒毛伸长和肠表面显著增加。作为跨代后果,肠道上皮缺乏Rank的雌性小鼠所生的婴儿在代谢应激后体重减轻并出现葡萄糖耐受不良。尽管妊娠/哺乳期肠道上皮重塑是可逆的,但活性形式RANK的组成性表达足以驱动肠道扩张,随后绒毛和干细胞的丢失,并阻止apcmin驱动的小肠干细胞肿瘤的形成。这些数据确定RANK-RANKL是妊娠/哺乳期间肠上皮扩张的途径,这是哺乳动物生活史和进化中最难以捉摸和最基本的组织重塑事件之一。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


