Dietary fructose enhances tumour growth indirectly via interorgan lipid transfer
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Fructose consumption has increased considerably over the past five decades, largely due to the widespread use of high-fructose corn syrup as a sweetener1. It has been proposed that fructose promotes the growth of some tumours directly by serving as a fuel2,3. Here we show that fructose supplementation enhances tumour growth in animal models of melanoma, breast cancer and cervical cancer without causing weight gain or insulin resistance. The cancer cells themselves were unable to use fructose readily as a nutrient because they did not express ketohexokinase-C (KHK-C). Primary hepatocytes did express KHK-C, resulting in fructolysis and the excretion of a variety of lipid species, including lysophosphatidylcholines (LPCs). In co-culture experiments, hepatocyte-derived LPCs were consumed by cancer cells and used to generate phosphatidylcholines, the major phospholipid of cell membranes. In vivo, supplementation with high-fructose corn syrup increased several LPC species by more than sevenfold in the serum. Administration of LPCs to mice was sufficient to increase tumour growth. Pharmacological inhibition of ketohexokinase had no direct effect on cancer cells, but it decreased circulating LPC levels and prevented fructose-mediated tumour growth in vivo. These findings reveal that fructose supplementation increases circulating nutrients such as LPCs, which can enhance tumour growth through a cell non-autonomous mechanism. Dietary fructose enhances tumour growth in animal models of melanoma, breast cancer and cervical cancer indirectly via metabolite transfer.
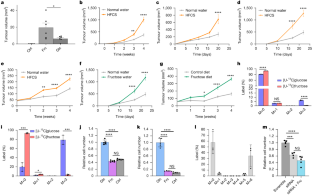

膳食果糖通过器官间脂质转移间接促进肿瘤生长
在过去的50年里,果糖的消耗量大幅增加,这主要是由于高果糖玉米糖浆作为甜味剂的广泛使用。有人提出果糖通过作为燃料直接促进某些肿瘤的生长2,3。本研究表明,在黑色素瘤、乳腺癌和宫颈癌的动物模型中,果糖补充剂可以促进肿瘤生长,而不会导致体重增加或胰岛素抵抗。癌细胞本身不能很容易地利用果糖作为营养物质,因为它们不表达酮己糖激酶c (KHK-C)。原代肝细胞确实表达KHK-C,导致果糖分解和多种脂类的排泄,包括溶血磷脂酰胆碱(LPCs)。在共培养实验中,肝细胞来源的LPCs被癌细胞消耗并用于生成磷脂酰胆碱,这是细胞膜的主要磷脂。在体内,补充高果糖玉米糖浆使血清中的几种LPC增加了7倍以上。给小鼠注射LPCs足以促进肿瘤生长。酮己糖激酶的药理抑制对癌细胞没有直接影响,但它可以降低循环中的LPC水平,并在体内阻止果糖介导的肿瘤生长。这些发现表明,补充果糖可以增加循环营养素,如LPCs,这可以通过细胞非自主机制促进肿瘤生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


