Pickering Emulsions: Role of Particle Wettability and Adhesive Force on Droplet Bridging
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
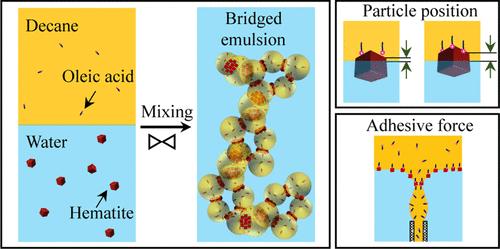
This study demonstrates the engineering of bridged Pickering emulsion (PE) gels by tuning the particle position at the interface and adhesive forces. This is achieved through controlled surface modification of hematite particles using oleic acid in a water–decane system. Microscopy observations revealed that the droplets are stabilized through a bridging mechanism, where oil droplets are connected by a shared monolayer of particles, with an intervening water layer between them. The experimental observations reveal that the concentration of oleic acid affects both the position of particles with respect to the interface (wettability) and adhesive forces, leading to the formation of emulsions with bridged droplets at specific oleic acid concentration ranges. To investigate this, the particle position at the interface and the strength of adhesive force are measured as a function of oleic acid concentration by direct visualization and droplet stretching technique, respectively. These studies confirm that at low oleic acid concentrations, the particle position favors the bridging, as particles are preferentially wettable by the continuous phase (water) but adhesive forces are not strong. Thus, this condition promotes the formation of oil-in-water emulsions without bridging. While, at higher oleic acid concentrations, the position of particles with respect to the interface hinders bridging, despite sufficient adhesive forces, because the particle surface becomes preferentially wettable by dispersed phase (oil), thereby supporting the inversion of emulsions. Therefore, a precise amount of oleic acid is necessary to achieve stable bridging with both factors contributing to the bridge formation. Further, the versatility of the process is illustrated by using different types of oil and particle surface modifiers. In all of the cases, stable emulsions are obtained by droplet bridging at a precise concentration of the modifier. The effect of the particle concentration and water-to-decane volume ratio on the stability of these emulsions is also studied. These emulsions show remarkable stability under undisturbed conditions due to gel-like nature despite the droplets being partially covered with particles. Moreover, after such emulsions are destabilized by external stimuli, emulsions with similar features can be readily and reversibly formed.
酸洗乳剂:颗粒润湿性和粘附力对液滴桥接的作用
本研究通过调整颗粒在界面处的位置和粘附力来证明桥接皮克林乳液(PE)凝胶的工程化。这是通过在水-癸烷体系中使用油酸对赤铁矿颗粒进行可控的表面改性来实现的。显微镜观察显示,油滴通过桥接机制稳定下来,其中油滴由共享的单层颗粒连接,中间有一个水层。实验结果表明,油酸浓度会影响颗粒相对于界面的位置(润湿性)和粘附力,导致在特定油酸浓度范围内形成具有桥接液滴的乳液。为了研究这一点,分别采用直接可视化和液滴拉伸技术测量了颗粒在界面处的位置和粘附力强度作为油酸浓度的函数。这些研究证实,在低油酸浓度下,颗粒位置有利于桥接,因为颗粒优先被连续相(水)润湿,但附着力不强。因此,这种情况促进了水包油乳液的形成,而没有桥接。然而,在较高的油酸浓度下,尽管有足够的粘附力,但颗粒相对于界面的位置阻碍了桥接,因为颗粒表面更容易被分散相(油)润湿,从而支持乳液的倒置。因此,精确的油酸量是实现桥接稳定的必要条件,这两种因素都有助于桥接的形成。此外,通过使用不同类型的油和颗粒表面改性剂,说明了该工艺的多功能性。在所有的情况下,稳定的乳液是通过液滴桥接在一个精确的浓度的改性剂。研究了颗粒浓度和水-癸烷体积比对乳剂稳定性的影响。尽管液滴部分被颗粒覆盖,但由于凝胶状性质,这些乳剂在未受干扰的条件下表现出显著的稳定性。此外,在这种乳剂被外部刺激破坏稳定后,具有类似特征的乳剂可以容易地和可逆地形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


