The GIP receptor activates futile calcium cycling in white adipose tissue to increase energy expenditure and drive weight loss in mice
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Obesity is a chronic disease that contributes to the development of insulin resistance, type 2 diabetes (T2D), and cardiovascular risk. Glucose-dependent insulinotropic polypeptide (GIP) receptor (GIPR) and glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) co-agonism provide an improved therapeutic profile in individuals with T2D and obesity when compared with selective GLP-1R agonism. Although the metabolic benefits of GLP-1R agonism are established, whether GIPR activation impacts weight loss through peripheral mechanisms is yet to be fully defined. Here, we generated a mouse model of GIPR induction exclusively in the adipocyte. We show that GIPR induction in the fat cell protects mice from diet-induced obesity and triggers profound weight loss (∼35%) in an obese setting. Adipose GIPR further increases lipid oxidation, thermogenesis, and energy expenditure. Mechanistically, we demonstrate that GIPR induction activates SERCA-mediated futile calcium cycling in the adipocyte. GIPR activation further triggers a metabolic memory effect, which maintains weight loss after the transgene has been switched off, highlighting a unique aspect in adipocyte biology. Collectively, we present a mechanism of peripheral GIPR action in adipose tissue, which exerts beneficial metabolic effects on body weight and energy balance.
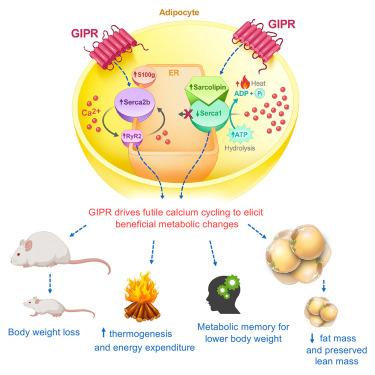
GIP受体激活白色脂肪组织中无用的钙循环,以增加能量消耗并推动小鼠体重减轻
肥胖是一种慢性疾病,会导致胰岛素抵抗、2型糖尿病(T2D)和心血管风险。与选择性GLP-1R激动作用相比,葡萄糖依赖性胰岛素性多肽(GIP)受体(GIPR)和胰高血糖素样肽-1 (GLP-1)受体(GLP-1R)共同激动作用可改善t2dm和肥胖患者的治疗效果。虽然GLP-1R激动作用的代谢益处已经确定,但GIPR激活是否通过外周机制影响体重减轻还没有完全确定。在这里,我们建立了一个仅在脂肪细胞中诱导GIPR的小鼠模型。我们发现,脂肪细胞中的GIPR诱导可以保护小鼠免受饮食诱导的肥胖,并在肥胖环境中引发体重减轻(约35%)。脂肪GIPR进一步增加脂质氧化、产热和能量消耗。在机制上,我们证明了GIPR诱导激活了脂肪细胞中serca介导的无效钙循环。GIPR激活进一步触发代谢记忆效应,在转基因关闭后保持体重减轻,突出了脂肪细胞生物学的一个独特方面。总之,我们提出了外周GIPR在脂肪组织中的作用机制,它对体重和能量平衡有有益的代谢作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


