Bifunctional carbon elimination mechanism of CeO2 nanorod@Ni3Co7Phy core shell catalyst for CO2 reforming of methane
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
CO2 capture and utilization via reforming of methane (DRM) is an efficient route to promote the achievement of the carbon neutralization goal. Whereas, the carbon deposition problem on cheap Ni-based catalysts slows down its industrial progress. Although Co doping to form NiCo bimetallic catalysts is effective to enhance the carbon resistance, the bifunctional carbon elimination mechanism has not been unveiled. Here, core shell structured CeO2@Ni7Co3Phy catalyst with high sintering and carbon resistance has been designed, exhibiting the stable CH4 and CO2 conversion of 72 % and 78 % respectively at 973 K for 120 h. The outstanding performance is due to the formation of Ni7Co3 bimetal, enhanced metal support interaction, and the highest surface Ni0 concentration, increasing sintering resistance and boosting the DRM activity. In situ diffuse infrared Fourier transform spectroscopy analysis and density functional theory calculations further confirm the bifunctional carbon elimination mechanism that both the oxygen vacancies in CeO2 and the electron deficient oxidative Co sites adsorb and activate CO2, providing oxygen species to eliminate carbon. By comparison, either serious Ni sintering and carbon accumulation or oxidation of Co0 phase due to excessive CO2 activation occurred for CeO2@NiPhy and CeO2@CoPhy catalyst respectively, leading to their worse DRM performance. The bifunctional carbon elimination mechanism illuminates the design of other carbon resistant catalysts.

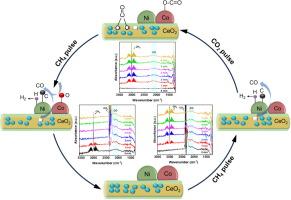
CeO2双功能碳消除机理nanorod@Ni3Co7Phy甲烷CO2重整核壳催化剂
甲烷重整技术(DRM)是促进碳中和目标实现的有效途径。然而,廉价镍基催化剂的积碳问题阻碍了其工业发展。虽然Co掺杂形成NiCo双金属催化剂能有效提高其抗碳性能,但其双功能除碳机理尚未明确。本文设计了具有高烧结性和抗碳性的核壳结构CeO2@Ni7Co3Phy催化剂,在973 K、120 h下,CH4和CO2的稳定转化率分别为72 %和78 %。优异的性能是由于形成了Ni7Co3双金属,增强了金属载体的相互作用,并且具有最高的表面Ni0浓度,增加了烧结阻力,提高了DRM活性。原位漫反射红外傅里叶变换光谱分析和密度泛函理论计算进一步证实了双功能碳消除机制,即CeO2中的氧空位和缺乏电子的氧化Co位点都吸附和激活CO2,为碳的消除提供了氧气。相比之下,CeO2@NiPhy和CeO2@CoPhy催化剂分别发生了严重的Ni烧结和碳堆积或Co0相氧化,导致其DRM性能较差。双功能碳消除机理对其他抗碳催化剂的设计具有指导意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


