Self-organization of the hematopoietic vascular niche and emergent innate immunity on a chip
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Here, we present a bioengineering approach to emulate the human bone marrow in vitro. Our developmentally inspired method uses self-organization of human hematopoietic stem and progenitor cells and vascular endothelial cells cultured in a three-dimensional microphysiological system to create vascularized, perfusable tissue constructs that resemble the hematopoietic vascular niche of the human marrow. The microengineered niche is capable of multilineage hematopoiesis and can generate functionally mature human myeloid cells that can intravasate into perfused blood vessels, providing a means to model the mobilization of innate immune cells from the marrow. We demonstrate the application of this system by presenting a specialized model of ionizing radiation-induced bone marrow injury and a multiorgan model of acute innate immune responses to bacterial lung infection. Furthermore, we introduce an advanced platform that enables large-scale integration and automated experimentation of the engineered hematopoietic tissues for preclinical screening of myelotoxicity due to anti-cancer drugs.
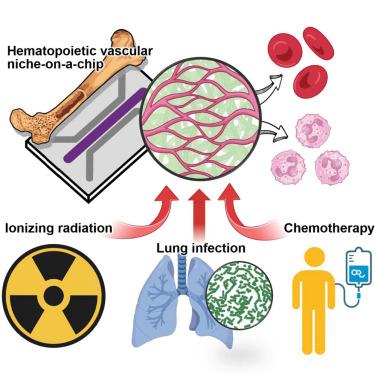
芯片上造血血管生态位的自组织与突发先天免疫
在这里,我们提出了一种生物工程方法来模拟体外的人类骨髓。我们的开发灵感的方法利用在三维微生理系统中培养的人类造血干细胞、祖细胞和血管内皮细胞的自组织来创建血管化的、可灌注的组织结构,类似于人类骨髓的造血血管生态位。微工程生态位能够多系造血,并能产生功能成熟的人骨髓细胞,这些细胞可以进入灌注血管,提供了一种模拟骨髓先天免疫细胞动员的方法。我们通过展示电离辐射诱导的骨髓损伤的专门模型和对细菌性肺部感染的急性先天免疫反应的多器官模型来演示该系统的应用。此外,我们还引入了一个先进的平台,可以大规模集成和自动化实验工程造血组织,用于抗癌药物引起的骨髓毒性的临床前筛选。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


