Sustainable protocol for Cu-catalysed A3-coupling under solvent-free conditions†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A Cu-catalyzed three-component cascade reaction has been developed, involving ortho-alkynylaryl aldehydes, terminal alkynes and aliphatic/aromatic amines or diamines. This diversity oriented methodology successfully delivered a rich library of 72 molecules in good to excellent yields (yields up to 99%) through the application of an A3-coupling reaction. This method is green, straightforward to execute, requires a short reaction time (2 min–4 h), does not require solvents or harsh or inert conditions, i.e. can be performed in open air, and utilizes only a small amount of a cheap and readily available catalyst (2.5 to 10 mol% CuI). It proficiently produced a variety of biphenylacetylene tethered propargylamines, alkyne tethered dihydroisoquinolines, and N-fused benzimidazoles with excellent regioselectivity.
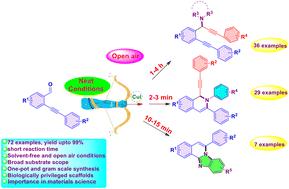
无溶剂条件下cu催化的a3偶联的可持续方案。
建立了一种铜催化的三组分级联反应,涉及邻炔芳基醛、末端炔和脂肪族/芳香胺或二胺。这种以多样性为导向的方法通过应用a3偶联反应成功地提供了72个分子的丰富文库,收率很高(收率高达99%)。该方法绿色环保,操作简单,反应时间短(2 min-4 h),不需要溶剂或苛刻或惰性条件,即可以在露天进行,并且只使用少量廉价且易得的催化剂(2.5至10 mol% CuI)。它熟练地生产各种联苯乙炔系链丙胺、炔系链二氢异喹啉和n -熔合苯并咪唑,具有优异的区域选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


