Effect of La Addition to Ceria on the Oxygen Storage Capacity and the Energetics of Water Adsorption
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
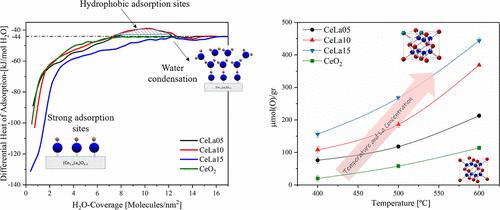
Ceria (CeO2) and doped ceria are well known for their catalytic surfaces that are active in various oxidation/reduction processes such as hydrogen production through thermochemical water splitting and three-way catalyst in combustion engines. Doping ceria with trivalent cations is expected to increase the concentration of oxygen vacancies due to charge compensation, but its effect on oxygen mobility or adsorption is not straightforward and depends on the specific trivalent element considered. In this study, we explore the effect of La addition on the bulk and surface properties of ceria by combining bulk (X-ray diffraction, thermogravimetry analysis, differential scanning calorimetry, and temperature-programmed desorption) and surface-sensitive techniques (X-ray photoelectron spectroscopy and water adsorption calorimetry). Three nanosized compositions of ceria doped with La were synthesized (at 5, 10, and 15% La3+) and thoroughly characterized. Compared with undoped ceria, the solid solutions obtained exhibited enhanced thermal stability. The solid solutions preserved their fluorite structure up to 1200 °C and exhibited a significantly reduced coarsening compared to pure ceria. The enhanced stability is attributed to the segregation of La to the surface. Doping of ceria with La led to an increase in the oxygen storage capacity, with the effect increasing with the increasing concentration of La. This increase was attributed to increased oxygen mobility with increasing La concentration. The addition of a small concentration of La (5%) leads to a significant increase in the amount of water adsorbed compared to pure ceria. Notably, water adsorption led to an enrichment of La on the surface, most pronounced for the highest La content, probably as the result of La diffusion from the subsurface to the surface. The heat of adsorption isotherms exhibits an unusual behavior, pointing to the need for further theoretical work.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


