New Insights into Thermal Degradation Products of Long-Chain Per- and Polyfluoroalkyl Substances (PFAS) and Their Mineralization Enhancement Using Additives
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
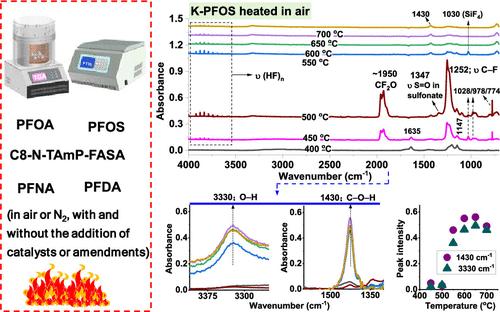
The products of incomplete destruction (PIDs) of per- and polyfluoroalkyl substances (PFAS) represent a substantial ambiguity when employing thermal treatments to remediate PFAS-contaminated materials. In this study, we present new information on PIDs produced in both inert and oxidative environments from five long-chain PFAS, including three now regulated under the U.S. Safe Drinking Water Act, one cationic precursor compound, and one C10 PFAS. The data did not support the generation of tetrafluoromethane from any of the studied PFAS, and carbonyl fluoride was found only from potassium perfluorooctanesulfonate (K-PFOS) when heated in air in a narrow temperature range. Oxidative conditions (air) were observed to facilitate PFAS thermal degradation and accelerate the mineralization of K-PFOS. Spectroscopic data suggest that PFAS thermal degradation is initiated by the cleavage of bonds that form perfluoroalkyl radicals, leading to organofluorine PIDs (e.g., perfluoroalkenes). In air, perfluoroalkyl radicals react with oxygen to form oxygen-containing PIDs. The mineralization of PFAS was enhanced by adding solid additives, which were categorized as highly effective (e.g., granular activated carbon (GAC) and certain noble metals), moderately effective, and noneffective. Remarkably, simply by adding GAC, we achieved >90% mineralization of perfluorooctanoic acid at 300 °C and ∼1.9 atm within just 60 min without using water or solvents.
长链全氟烷基和多氟烷基物质(PFAS)热降解产物及其添加剂强化矿化的新认识
全氟烷基和多氟烷基物质(PFAS)的不完全破坏产物(PIDs)在采用热处理修复PFAS污染的材料时具有很大的模糊性。在这项研究中,我们介绍了五种长链PFAS在惰性和氧化环境下产生的pid的新信息,其中包括三种目前受美国安全饮用水法案监管的PFAS,一种阳离子前体化合物和一种C10 PFAS。数据不支持从所研究的任何一种全氟化合物中产生四氟甲烷,而且羰基氟仅在全氟辛烷磺酸钾(K-PFOS)中在狭窄温度范围内空气加热时发现。氧化条件(空气)有利于PFAS的热降解,加速K-PFOS的矿化。光谱数据表明,PFAS的热降解是由形成全氟烷基自由基的键的断裂引起的,导致有机氟PIDs(例如,全氟烯烃)。在空气中,全氟烷基自由基与氧反应形成含氧环氧丙烷。添加固体添加剂可增强PFAS的矿化,固体添加剂分为高效(如颗粒活性炭(GAC)和某些贵金属)、中等有效和无效。值得注意的是,仅通过添加GAC,我们就在300°C和~ 1.9 atm的条件下,在60分钟内实现了全氟辛酸90%的矿化,而无需使用水或溶剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


