Engineered Cyclotide Blocks Neuronal Excitotoxicity
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Cyclotides are naturally occurring cyclic peptides with three disulfide bonds, offering remarkable stability. In neurological disorders, the formation of a complex between postsynaptic density protein 95 and NMDA receptors (NMDARs) can lead to neuronal cell death. In this study, we modified the MCoTI-II cyclotide backbone with polyarginines for enhanced intracellular delivery and grafted a 9-amino acid PSD-95-NMDAR inhibitor sequence, NR2B9c, into loop 6. We found that incorporating polyarginines into the cyclotide backbone significantly improved uptake into neuronal cells. Primary neurons treated with the NR2B9c cyclotide (c5R-NR2B9c) prevented cell death in response to high concentrations of N-methyl-d-aspartate (NMDA), demonstrating protection from excitotoxicity. Administration of c5R-NR2B9c in a chemically induced seizure model in mice resulted in increased survival and reduced seizure severity. Overall, we show that modifying cyclotides with a polyarginine backbone can enhance the delivery of therapeutic peptides into neuronal cells, which can be utilized to administer therapeutic peptides for the protection of neuronal cells from excitotoxicity.
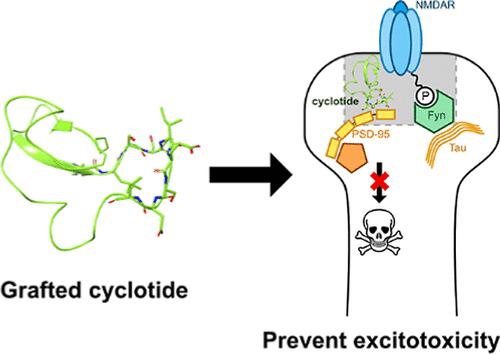
工程环肽阻断神经元兴奋性毒性
环肽是天然存在的具有三个二硫键的环状肽,具有显著的稳定性。在神经系统疾病中,突触后密度蛋白95和NMDA受体(NMDARs)之间复合物的形成可导致神经元细胞死亡。在这项研究中,我们用聚精氨酸修饰MCoTI-II环肽骨架以增强细胞内递送,并将9个氨基酸的PSD-95-NMDAR抑制剂序列NR2B9c移植到环6中。我们发现,将聚精氨酸结合到环肽骨架中可以显著改善神经元细胞的摄取。用NR2B9c环肽(c5R-NR2B9c)处理的原代神经元可以防止高浓度n -甲基-d-天冬氨酸(NMDA)的细胞死亡,显示出对兴奋性毒性的保护作用。c5R-NR2B9c在小鼠化学诱导癫痫模型中可提高存活率,降低癫痫发作严重程度。总的来说,我们发现用聚精氨酸骨架修饰环肽可以增强治疗肽向神经元细胞的传递,这可以用于管理治疗肽以保护神经元细胞免受兴奋性毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


