Unveiling the Antimycobacterial Potential of Novel 4-Alkoxyquinolines: Insights into Selectivity, Mechanism of Action, and In Vivo Exposure
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
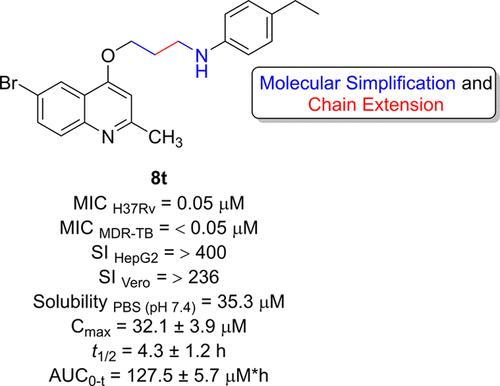
This work presents a comprehensive investigation into the design, synthesis, and evaluation of a novel series of 4-alkoxyquinolines as potential antimycobacterial agents. The design approach, which combined molecular simplification and chain extension, resulted in compounds with potent and selective activity against both drug-susceptible and multidrug-resistant Mycobacterium tuberculosis strains. The lead molecule, targeting the cytochrome bc1 complex, exhibited favorable kinetic solubility and remarkable chemical stability under acidic conditions. Despite in vitro ADME evaluations showing low permeability and high metabolism in rat microsomes, the lead compound exhibited bacteriostatic activity in a murine macrophage model of TB infection and demonstrated promising in vivo exposure following gavage in mice, with an AUC0–t of 127.5 ± 5.7 μM h. To the best of our knowledge, for the first time, a simplified structure from 2-(quinolin-4-yloxy)acetamides has shown such potential. These findings suggest a new avenue for exploring this chemical class as a source of antituberculosis drug candidates.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


