Discovery of Potent and Selective Pyridone-Based Small Molecule Kinetic Stabilizers of Amyloidogenic Immunoglobulin Light Chains
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Kinetic stabilization of amyloidogenic immunoglobulin light chains (LCs) through small molecule binding may become the first treatment for the proteinopathy component of light chain amyloidosis (AL). Kinetic stabilizers selectively bind to the native state over the misfolding transition state, slowing denaturation. Prior λ full-length LC dimer (FL LC2) kinetic stabilizers exhibited considerable plasma protein binding. We hypothesized that the coumarin “aromatic core” of the stabilizers was responsible for the undesirable plasma protein binding. Here, we describe structure–activity relationship (SAR) data initially focused on replacing the coumarin aromatic core. 2-pyridones proved suitable replacements. We subsequently optimized the “anchor substructure” in the context of 2-pyridones, resulting in potent λ FL LC2 kinetic stabilizers exhibiting reduced plasma protein binding. The 3-methyl- or 3-ethyl-3-phenylpyrrolidine–2-pyridone scaffold stabilized multiple AL patient-derived λ FL LC2s in human plasma. This, coupled with X-ray crystallographic data, indicates that 3-alkyl-3-phenylpyrrolidine–2-pyridone-based stabilizers are promising candidates for treating the proteinopathy component of AL.
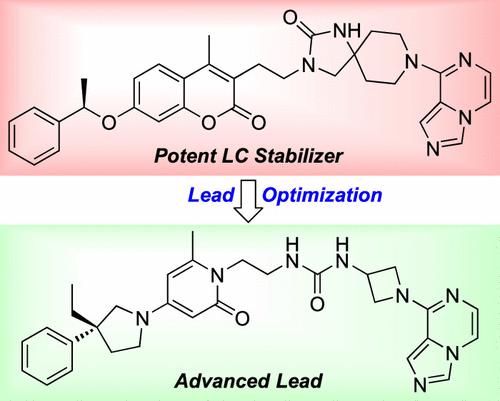
基于吡啶酮的淀粉样蛋白轻链小分子动力学稳定剂的发现
通过小分子结合实现淀粉样原性免疫球蛋白轻链(LCs)的动力学稳定可能成为治疗轻链淀粉样变性(AL)蛋白病变成分的首选方法。动态稳定剂选择性地结合原生状态,而不是错误折叠的过渡状态,减缓变性。先前的λ全长LC二聚体(FL LC2)动力学稳定剂表现出相当大的血浆蛋白结合。我们假设稳定剂的香豆素“芳香核”是导致不良血浆蛋白结合的原因。在这里,我们描述的构效关系(SAR)数据最初集中在取代香豆素芳香族核心。2-吡啶酮被证明是合适的替代品。我们随后在2-吡啶酮的背景下优化了“锚定亚结构”,得到了有效的λ FL LC2动力学稳定剂,显示出血浆蛋白结合减少。3-甲基或3-乙基-3-苯基吡咯烷- 2-吡啶酮支架稳定了人血浆中多个AL患者来源的λ FL LC2s。这与x射线晶体学数据相结合,表明3-烷基-3-苯基吡咯烷- 2-吡啶基稳定剂是治疗AL蛋白病变成分的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


