Scale-Up of Continuous Metallaphotoredox Catalyzed C–O Coupling to a 10 kg-Scale Using Small Footprint Photochemical Taylor Vortex Flow Reactors
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
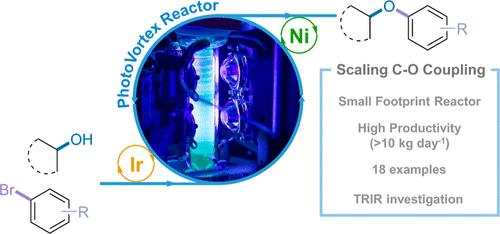
We report the development and optimization of a scalable flow process for metallaphotoredox (Ir/Ni) C–O coupling, a mild and efficient approach for forming alkyl-aryl ethers, a common motif in medicinal and process chemistry settings. Time-resolved infrared spectroscopy (TRIR) highlighted the amine as the major quencher of the photocatalyst triplet excited state, along with the formation of an Ir(II) species that, in the presence of the Ni cocatalyst, has its lifetime shortened, suggesting reductive quenching of Ir(III)*, followed by reoxidation facilitated by the Ni cocatalyst. TRIR and batch reaction screening was used to develop conditions transferrable to flow, and many processing benefits of performing the reaction in flow were then demonstrated using a simple to construct/operate, small-footprint FEP coil flow reactor, including short (<10 min) space times and reduced catalyst loadings (down to 0.1 mol % Ir, 1 mol % Ni) while retaining good yield/conversion. Scalability was demonstrated by increasing the length or diameter of the FEP coil flow reactor tubing, however, due to suspected mass transfer/mixing limitations, the yield decreased upon scale-up in some cases. Therefore, we applied a modified version of our previously reported photochemical Taylor Vortex Flow Reactor (PhotoVortex), where Taylor vortices and a short-irradiated path length allow photochemical reactions to be performed efficiently via excellent mixing. In a small PhotoVortex (8 mL irradiated volume), we have demonstrated projected productivities around 1 kg day–1 and >10 kg day–1 in a large PhotoVortex (185 mL irradiated volume) with good product yields (>90%) and low catalyst loadings (0.1 to 0.5 mol % of [Ir{dF(CF3)ppy}2dtbbpy]PF6), enabled by excellent mixing ensuring sufficient mass transfer between short-lived photoexcited and other transient species.
利用小足迹光化学泰勒涡流反应器将连续金属氧化还原催化的C-O偶联放大到10公斤级
我们报告了金属光氧化还原(Ir/Ni) C-O偶联的可扩展流程的开发和优化,这是一种温和而有效的形成烷基芳基醚的方法,是医学和工艺化学设置中的常见主题。时间分辨红外光谱(TRIR)显示,胺是光催化剂三重态激发态的主要猝灭剂,同时形成Ir(II),在Ni助催化剂的存在下,Ir(III)*的寿命缩短,表明Ir(III)*的还原性猝灭,随后Ni助催化剂促进了再氧化。TRIR和间歇反应筛选用于开发可转移到流动的条件,然后使用简单的构造/操作,占地面积小的FEP线圈流反应器演示了在流动中进行反应的许多处理优势,包括短(<;10分钟)的空间时间和减少催化剂负载(低至0.1 mol % Ir, 1 mol % Ni),同时保持良好的收率/转化率。可扩展性通过增加FEP线圈流反应器管的长度或直径来证明,然而,由于怀疑的传质/混合限制,在某些情况下,放大后的产率会下降。因此,我们采用了先前报道的光化学泰勒涡流反应器(PhotoVortex)的改进版本,其中泰勒涡流和较短的辐照路径长度允许通过良好的混合有效地进行光化学反应。在一个小的PhotoVortex (8ml辐照体积)中,我们已经证明了在一个大的PhotoVortex (185 mL辐照体积)中,预计的生产力约为1kg day-1和10kg day-1,具有良好的产品收率(>90%)和低催化剂负载(0.1 - 0.5 mol %的[Ir{dF(CF3)ppy}2dtbbpy]PF6),通过良好的混合确保了短寿命光激发和其他瞬态物质之间的充分传质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


