Concerted methane fixation at ambient temperature and pressure mediated by an alcohol oxidase and Fe-ZSM-5 catalytic couple
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Anthropogenic methane emissions, particularly from diffuse and dilute sources, pose a significant challenge for oxidation and valorization as existing methane oxidation routes rely on high temperatures or pressures. Here we report the catalytic coupling of alcohol oxidase with the iron-modified ZSM-5 (Fe-ZSM-5) zeolite catalyst, creating a tandem methanotrophic system that partially oxidizes methane at ambient temperatures and pressures. Methane reacts at Fe-ZSM-5 to produce methanol, which is then oxidized at the enzyme to formaldehyde and hydrogen peroxide. The latter subsequently reacts back at Fe-ZSM-5 and oxidizes methane in a catalytic couple. We show that methane-to-formaldehyde selectivity can exceed 90% at room temperature. The generated formaldehyde was rapidly incorporated into a growing urea polymer, with a material growth rate exceeding 5.0 mg gcat−1 h−1, which matches or exceeds the growth rates of many methanotrophic organisms. This work presents a sustainable route for methane oxidation, driven by oxygen in the air under ambient conditions, producing high-value polymers and valorizing methane emission streams. The development of catalytic systems for sequestering anthropogenic methane emissions from the atmosphere could potentially reduce global warming. Now, coupling the enzyme alcohol oxidase with an inorganic zeolite generates formaldehyde from methane under ambient conditions with 90% selectivity.
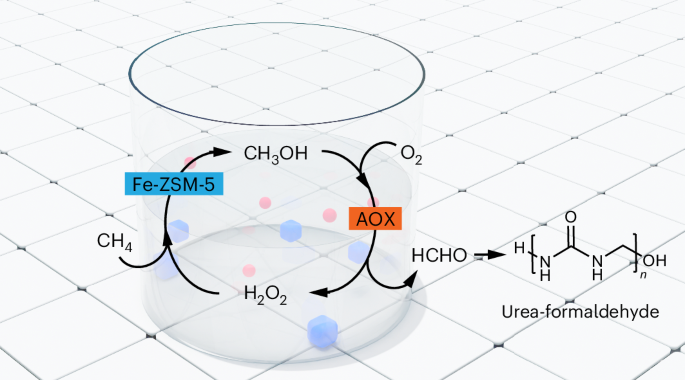

乙醇氧化酶和Fe-ZSM-5催化偶联介导的常温常压甲烷协同固定
由于现有的甲烷氧化途径依赖于高温或高压,人为甲烷排放,特别是来自扩散和稀释源的甲烷排放,对氧化和增值构成了重大挑战。本文报道了醇氧化酶与铁修饰的ZSM-5 (Fe-ZSM-5)沸石催化剂的催化偶联,创建了一个串联的甲烷氧化系统,该系统在室温和常压下部分氧化甲烷。甲烷在Fe-ZSM-5上反应生成甲醇,然后在酶下氧化为甲醛和过氧化氢。后者随后与Fe-ZSM-5反应并在催化偶对中氧化甲烷。我们表明,在室温下,甲烷对甲醛的选择性可以超过90%。生成的甲醛迅速融入到生长的尿素聚合物中,材料生长速度超过5.0 mg gcat - 1 h - 1,与许多甲烷化生物的生长速度相当或超过。这项工作提出了一种可持续的甲烷氧化途径,在环境条件下由空气中的氧气驱动,产生高价值聚合物并使甲烷排放流增值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


