Type I IFN-mediated NET release promotes Mycobacterium tuberculosis replication and is associated with granuloma caseation
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Neutrophils are the most abundant cell type in the airways of tuberculosis patients. Mycobacterium tuberculosis (Mtb) infection induces the release of neutrophil extracellular traps (NETs); however, the molecular regulation and impact of NET release on Mtb pathogenesis are unknown. We find that during Mtb infection in neutrophils, PAD4 citrullinates histones to decondense chromatin that gets released as NETs in a manner that can maintain neutrophil viability and promote Mtb replication. Type I interferon promotes the formation of chromatin-containing vesicles that allow NET release without compromising plasma membrane integrity. Analysis of nonhuman primate granulomas supports a model where neutrophils are exposed to type I interferon from macrophages as they migrate into the granuloma, thereby enabling the release of NETs associated with necrosis and caseation. Our data reveal NET release as a promising target to inhibit Mtb pathogenesis.
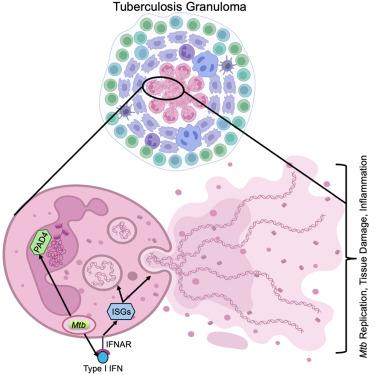
I型ifn介导的NET释放促进结核分枝杆菌复制并与肉芽肿干酪化有关
嗜中性粒细胞是肺结核患者气道中最丰富的细胞类型。结核分枝杆菌(Mtb)感染诱导中性粒细胞胞外陷阱(NETs)的释放;然而,NET释放对结核分枝杆菌发病机制的分子调控和影响尚不清楚。我们发现,在中性粒细胞感染Mtb期间,PAD4瓜氨酸化组蛋白去致密染色质,以net的方式释放,可以维持中性粒细胞活力并促进Mtb复制。I型干扰素促进含染色质囊泡的形成,使NET释放而不损害质膜的完整性。对非人灵长类动物肉芽肿的分析支持一种模型,即当巨噬细胞迁移到肉芽肿时,中性粒细胞暴露于来自巨噬细胞的I型干扰素,从而使与坏死和干酪化相关的NETs释放。我们的数据显示,NET释放是抑制结核分枝杆菌发病机制的一个有希望的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


