Cytoadhesion of Plasmodium falciparum-Infected Red Blood Cells Changes the Expression of Cytokine-, Histone- and Antiviral Protein-Encoding Genes in Brain Endothelial Cells
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
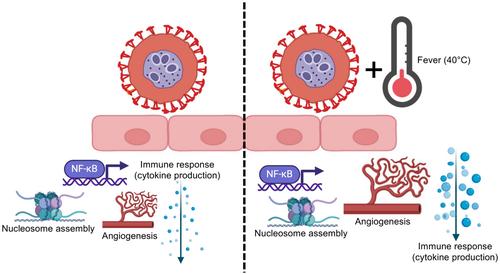
Malaria remains a significant global health problem, mainly due to Plasmodium falciparum, which is responsible for most fatal infections. Infected red blood cells (iRBCs) evade spleen clearance by adhering to endothelial cells (ECs), triggering capillary blockage, inflammation, endothelial dysfunction and altered vascular permeability, prompting an endothelial transcriptional response. The iRBCIT4var04/HBEC-5i model, where iRBCs present IT4var04 (VAR2CSA) on their surface, was used to analyze the effects of iRBC binding on ECs at different temperature (37°C vs. 40°C). Binding of non-infected RBCs (niRBCs) and fever alone altered the expression of hundreds of genes in ECs. Comparing the expression profile of HBEC-5i cells cultured either in the presence of iRBCs or in the presence of niRBCs revealed significant upregulation of genes linked to immune response, nucleosome assembly, NF-kappa B signaling, angiogenesis, and antiviral immune response/interferon-alpha/beta signaling. Raising the temperature to 40°C, simulating fever, led to further upregulation of many genes, particularly those involved in cytokine production and angiogenesis. In summary, the presence of iRBCs stimulates ECs, activating several immunological pathways and affecting antiviral (−parasitic) mechanisms and angiogenesis. Our data uncovered the induction of the interferon-alpha/beta signaling pathway in ECs in response to iRBCs.
恶性疟原虫感染红细胞的细胞粘附改变脑内皮细胞细胞因子、组蛋白和抗病毒蛋白编码基因的表达
疟疾仍然是一个重大的全球健康问题,主要原因是恶性疟原虫,它是大多数致命感染的罪魁祸首。受感染的红细胞(irbc)通过粘附内皮细胞(ECs)逃避脾脏清除,引发毛细血管阻塞、炎症、内皮功能障碍和血管通透性改变,引发内皮转录反应。iRBCIT4var04/HBEC-5i模型,iRBC在其表面存在IT4var04 (VAR2CSA),用于分析不同温度(37℃vs. 40℃)下iRBC结合对ECs的影响。非感染红细胞(nirbc)与发热单独结合改变了ECs中数百个基因的表达。比较在irbc或nirbc存在下培养的hbc -5i细胞的表达谱,发现与免疫反应、核小体组装、nf - κ B信号传导、血管生成和抗病毒免疫反应/干扰素- α / β信号传导相关的基因显著上调。将温度升高到40°C,模拟发烧,导致许多基因的进一步上调,特别是那些与细胞因子产生和血管生成有关的基因。总之,irbc的存在刺激内皮细胞,激活几种免疫途径,影响抗病毒(-寄生)机制和血管生成。我们的数据揭示了干扰素- α / β信号通路在内皮细胞中对irbc的响应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


