Mo4+-Pb2+ redox triggers self-enhanced removal and recovery of Pb2+ with superselectivity
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Membrane-based electrodeposition (MED) has emerged as a promising approach for reversible removal-recovery of toxic but valuable Pb2+. However, limited by the low specificity of membrane deposition toward various heavy metal ions in MED, the selective removal of Pb2+ remains an obstacle. Inspired by the soft-hard acid-base theory, here we developed a Pb2+-affinity electroactive membrane by incorporating MoS2 with the cation exchange membrane (CEM) to achieve a tandem Pb2+ selective adsorption-deposition process. CEM@MoS2 achieved nearly 100 % Pb2+ removal selectivity even in the presence of diverse competing cations, with a remarkable distribution coefficient (1.3 × 107 mL·g−1) and treatment capacity (2580.4 mg·g−1), resulting in a high-purity Pb2+ concentrate recovery. Importantly, a spontaneous Mo4+-Pb2+ redox reaction was found, which triggered Pb2+ reduction to metallic Pb. This surficial Pb° formation decreased the energy barrier for subsequent membrane H2O splitting and Pb2+ reduction, accounting for the unexpected self-enhanced Pb2+ removal scenario. Additionally, the exhausted Mo4+ species was facilely regenerated via a cathodic reduction method, demonstrating excellent stability and reusability. The work is expected to provide a viable strategy for selective removal-recovery of heavy metal ions using MED.

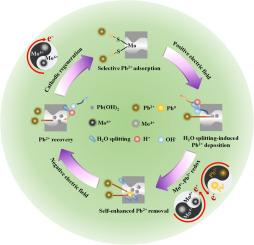
Mo4+-Pb2+氧化还原触发超选择性自增强Pb2+的去除和恢复
膜基电沉积(MED)已成为一种很有前途的方法,用于有毒但有价值的Pb2+的可逆去除和回收。然而,由于膜沉积对MED中各种重金属离子的特异性较低,Pb2+的选择性去除仍然是一个障碍。受软硬酸碱理论的启发,我们将MoS2与阳离子交换膜(CEM)结合,开发了Pb2+亲和电活性膜,实现了Pb2+的串联选择性吸附-沉积过程。在不同竞争阳离子存在的情况下,CEM@MoS2对Pb2+的去除率接近100%,具有显著的分布系数(1.3 × 107 mL·g−1)和处理量(2580.4 mg·g−1),可回收高纯度Pb2+精矿。重要的是,发现了一个自发的Mo4+-Pb2+氧化还原反应,触发了Pb2+还原成金属Pb。这种表面Pb°的形成降低了随后膜水分裂和Pb2+还原的能量垒,导致了意想不到的自增强Pb2+去除情况。此外,耗尽的Mo4+物质通过阴极还原方法很容易再生,表现出优异的稳定性和可重复使用性。这项工作有望为MED选择性去除-回收重金属离子提供一种可行的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


