Enhanced Rapid and Efficient Recycling of Lithium-Ion Battery Cathode by Synergistic Effects of Ternary Deep Eutectic Solvents ChCl/MClx/Levulinic Acid
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The rapid growth of spent lithium-ion batteries (LIBs) raises concerns over the supply chain of critical metals and environmental impacts, emphasizing the urgent need for efficient recycling technologies. Due to their potential to reduce the consumption of energy and avoid the use of corrosive acid, deep eutectic solvents (DESs) have been widely studied to selectively leach and recover valuable metals from spent LIBs. There is general agreement that DESs with high acidity, coordination, reducibility, and low viscosity could efficiently dissolve cathode materials. However, it is hard to design binary DES compositions to fulfill all of these requirements simultaneously. Herein, this study focuses on the design of ternary DESs, leveraging the synergistic effects of their components to achieve strong coordination and low viscosity, enhanced acidity, and reducibility to efficiently extract valuable metals. Specifically, ChCl/CuCl:4Levulinic acid demonstrated exceptional leaching capabilities, recovering over 90% of LiCoO2 within 10 min. Furthermore, to ensure the purity of recovered metal products, ChCl/LiCl:8Levulinic acid was investigated to realize the efficient leaching, recovery, and DES regeneration process. It was verified that ChCl/LiCl/8Levulinic acid has high leaching efficiency and good cycling stability and facilitates a convenient recycling process. Overall, this work demonstrates the potential of tailored ternary DESs for the efficient leaching of cathode materials and proposes a sustainable process to realize metal recovery from spent LIB cathode and DES regeneration.
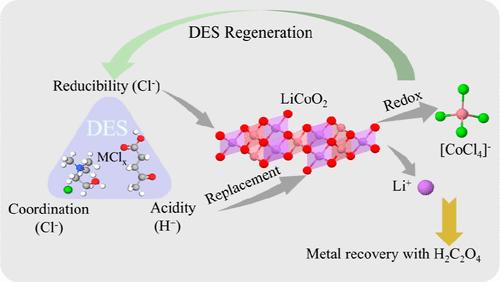
三元深共晶溶剂ChCl/MClx/乙酰丙酸协同作用增强锂离子电池正极的快速高效回收
废旧锂离子电池(lib)的快速增长引发了对关键金属供应链和环境影响的担忧,强调了对高效回收技术的迫切需要。由于具有降低能耗和避免使用腐蚀性酸的潜力,深度共晶溶剂(DESs)被广泛研究用于选择性浸出和回收废lib中的有价金属。人们普遍认为,高酸度、高配位性、高还原性和低粘度的DESs能有效地溶解正极材料。然而,很难设计出同时满足所有这些要求的二元DES组合。为此,本研究重点设计三元DESs,利用其组分的协同作用,实现强配位,低粘度,增强酸度,还原性,高效提取有价金属。具体而言,ChCl/CuCl: 4levulic酸表现出优异的浸出能力,在10分钟内回收超过90%的LiCoO2。此外,为了确保回收金属产品的纯度,研究了ChCl/LiCl: 8levulic酸,以实现高效的浸出,回收和DES再生过程。验证了ChCl/LiCl/8乙酰丙酸浸出效率高,循环稳定性好,回收工艺方便。总的来说,这项工作证明了定制三元DESs在阴极材料有效浸出方面的潜力,并提出了一种可持续的工艺,可以实现从废LIB阴极和DES再生中回收金属。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
文献相关原料
公司名称
产品信息
麦克林
zinc chloride (ZnCl2)
麦克林
ferric chloride (FeCl3)
麦克林
zinc chloride (ZnCl2)
麦克林
ferric chloride (FeCl3)
麦克林
zinc chloride (ZnCl2)
麦克林
ferric chloride (FeCl3)
百灵威
iron(III) chloride hexahydrate (FeCl3·6H2O)
百灵威
lithium chloride (LiCl)
百灵威
iron(III) chloride hexahydrate (FeCl3·6H2O)
百灵威
lithium chloride (LiCl)
阿拉丁
cobalt chloride hexahydrate (CoCl2·6H2O)
阿拉丁
cobalt chloride hexahydrate (CoCl2·6H2O)
阿拉丁
cobalt chloride hexahydrate (CoCl2·6H2O)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


