Engineering Interference-Removal and Target-Response Cascade Zones on Wood Channels for Straightforward Detection of Uric Acid in Saliva
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
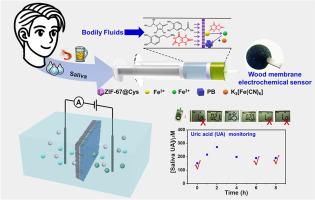
The detection of biomarkers is crucial for assessing disease status and progression. Uric acid (UA), a common biomarker in body fluids, plays an important role in the diagnosis and monitoring of conditions such as hyperuricemia, chronic kidney disease, and cardiovascular disease. However, the low concentration of UA in non-invasive body fluids, combined with numerous interfering substances, makes its detection challenging. Therefore, there is a pressing need to develop advanced sensor platforms that exhibit both high sensitivity and excellent specificity for accurate monitoring of UA levels in various body fluids. In this study, an electrochemical sensing system is developed by integrating a cascade interference-removal zone and a target-response zone on a wooden channel membrane (CM) for the pretreatment-free detection of UA in body fluids. In this design, cysteine-doped ZIF-67(Co) nanocrystals, a mimic with multi-enzyme activity, are modified in the interference-removal zone. The target-response zone on the other side of the CM is contacted with a solution containing Fe3+ and [Fe(CN)6]3– ions. Using saliva as a proof-of-concept, the interference species, including ascorbic acid (AA), dopamine (DA), and glutathione (GSH), are oxidized and removed when passing through the interference-removal zone, while UA reaches the target-response zone and triggers the growth of Prussian blue (PB) on site. Utilizing the peroxidase-like activity of PB, UA concentration is directly determined based on changes in transmembrane ion currents. The resulting sensing system exhibits higher sensitivity than commercial UA meters and demonstrates straightforward, continuous, and non-invasive monitoring of UA in saliva after consuming purine-rich foods. The technology provides a simple, reliable and low-cost device for sensing UA in complex biological matrices. Moreover, the CM based sensing device also provides the benefit of being incineratable and biodegradable, reducing medical and electronic waste, making it eco-friendly for daily diagnostics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


