Unveiling the practical impact of cyclic additive- ethyl-4-toluene sulfonate on stable interface and extended lifespan using a combination of theory and experiments in full cell LIBs
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Additives play a pivotal role in enhancing lithium-ion battery performance, safety, and lifespan by mitigating electrolyte degradation, improving ion transport, and stabilizing the SEI layer to address issues of thermal instability and dendritic growth. This study evaluates Ethyl-4-toluene sulfonate (ETS) as a promising cyclic additive for improving lithium-ion battery efficiency and longevity. Through a combination of theoretical and experimental analyses, we assessed the stability and electrochemical properties of SEI layers with ETS integration.
Our results show that ETS strongly interacts with electrode surfaces, fostering stable SEI formation and substantially reducing electrolyte oxidation. Highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy analyses indicate that ETS oxidizes prior to carbonate solvents, contributing to enhanced structural integrity. Adsorption energy calculations further reveal that ETS adheres to electrode surfaces more effectively than traditional electrolytes, reinforcing SEI stability.
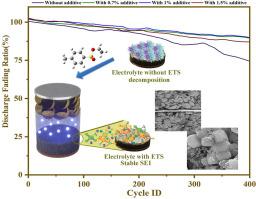
Experimental evaluations using FT-IR, XRD, and Rietveld refinement characterized electrode structure changes pre- and post-cycling. Testing electrolytes composed of ethylene carbonate (EC), dimethyl carbonate (DMC), and LiPF6 with varied ETS concentrations, we found that the sample with 0.7 % ETS exhibited optimal stability, achieving a high capacity of 1609.559 mAh.g⁻¹ over 400 cycles. SEM and impedance measurements confirmed substantial improvements in electrode structure and reduced resistance. The ETS-free electrolyte retained only 74.08 % capacity after 400 cycles and failed after 610 cycles, whereas the 0.7 % ETS sample maintained 90.55 % capacity and lasted approximately 920 cycles. These findings underscore the potential of ETS to enhance SEI formation, prevent electrolyte degradation, and improve battery performance, positioning ETS as a valuable additive for advanced lithium-ion batteries.

利用理论与实验相结合的方法揭示环添加剂-4-甲苯磺酸乙酯对全细胞lib稳定界面和延长寿命的实际影响
添加剂在提高锂离子电池的性能、安全性和寿命方面发挥着关键作用,它们可以减轻电解质降解、改善离子传输、稳定SEI层,从而解决热不稳定性和枝晶生长问题。本研究评价了乙基-4-甲苯磺酸盐(ETS)作为一种有前途的循环添加剂,可以提高锂离子电池的效率和寿命。通过理论和实验相结合的分析,我们评估了与ETS集成的SEI层的稳定性和电化学性能。我们的研究结果表明,ETS与电极表面强烈相互作用,促进稳定的SEI形成,并大大减少电解质氧化。最高已占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)能量分析表明,ETS在碳酸盐溶剂之前氧化,有助于增强结构完整性。吸附能计算进一步表明,ETS比传统电解质更有效地附着在电极表面,增强了SEI的稳定性。实验评价采用FT-IR, XRD和Rietveld细化表征电极结构的变化前后循环。通过测试不同ETS浓度的碳酸乙酯(EC)、碳酸二甲酯(DMC)和LiPF6组成的电解质,我们发现含有0.7% ETS的样品具有最佳的稳定性,达到1609.559 mAh的高容量。G - 1超过400个周期。扫描电镜和阻抗测量证实了电极结构的实质性改进和电阻的降低。无ETS的电解质在400次循环后仅保持74.08%的容量,在610次循环后失效,而0.7%的ETS样品保持90.55%的容量,持续约920次循环。这些发现强调了ETS在促进SEI形成、防止电解质降解和提高电池性能方面的潜力,使ETS成为先进锂离子电池的有价值的添加剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


