Three-dimensional nanoflower-like transition metal sulfide heterostructures (Co9S8/Co1-xS /WS2) as efficient bifunctional oxygen electrocatalysts for Zn-air batteries
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
The charging and discharging processes of rechargeable Zn-air batteries (ZABs) require the use of precious metal catalysts (Pt and RuO2) that are expensive. Therefore, preparing efficient, stable, and affordable metal bifunctional oxygen electrocatalysts compatible with both the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) is crucial. Herein, a nanoflower-like Co9S8/Co1-xS/WS2 bifunctional oxygen electrocatalyst with a layered structure has been prepared. The cobalt sulfide serves as the main active site and the synergistic interaction between the multi-components modulates the electronic structure of the active site and generates abundant S vacancies, thereby effectively improving the bifunctional oxygen electrocatalytic performance. The electrocatalytic activity of the prepared catalyst for ORR/OER is close to that of commercial noble metal catalysts. When Co9S8/Co1-xS/WS2 is used as the cathode material in Zn-air batteries, the batteries exhibit high specific capacity (822.6 mAh gZn−1), excellent energy density (958.9 Wh kgZn−1), as well as a battery charge/discharge cycling time that was twice that of the commercial catalyst PtC+RuO2.
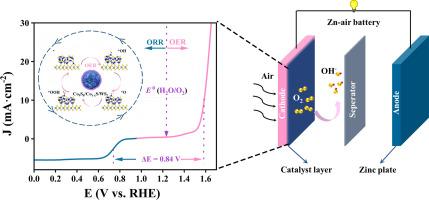

三维纳米花状过渡金属硫化物异质结构(Co9S8/Co1-xS /WS2)作为锌空气电池高效双功能氧电催化剂
可充电锌空气电池(ZABs)的充放电过程需要使用昂贵的贵金属催化剂(Pt和RuO2)。因此,制备高效、稳定、经济的金属双功能氧电催化剂,同时兼容氧还原反应(ORR)和析氧反应(OER)是至关重要的。本文制备了具有层状结构的纳米花状Co9S8/Co1-xS/WS2双功能氧电催化剂。硫化钴作为主要活性位点,多组分之间的协同作用调节了活性位点的电子结构,产生丰富的S空位,从而有效地提高了双官能团氧电催化性能。制备的ORR/OER催化剂的电催化活性与市售贵金属催化剂接近。当Co9S8/Co1-xS/WS2作为正极材料用于锌空气电池时,电池具有较高的比容量(822.6 mAh gZn−1),优异的能量密度(958.9 Wh kgZn−1),电池充放电循环时间是商用催化剂PtC+RuO2的两倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
文献相关原料
公司名称
产品信息
阿拉丁
Dimethylimidazole
阿拉丁
Methanol
阿拉丁
Thiourea
阿拉丁
Phosphotungstic acid
阿拉丁
Dimethylimidazole
阿拉丁
methanol
阿拉丁
thiourea
阿拉丁
phosphotungstic acid
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


