Fast ionic conduction achieved through the design and synthesis of ceramic heterointerfaces
IF 38.6
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
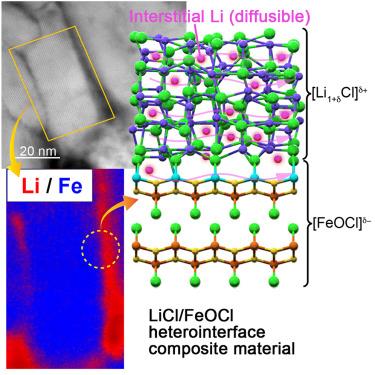
Lithium (Li) chloride and iron oxychloride (FeOCl), typically nonconductive, were combined to form a [Li1+δCl]δ+/[FeOCl]δ− heterointerface composite material (LFH), achieving ionic conductivities of >1 mS cm−1. Analysis techniques (scanning transmission electron microscopy [STEM] and electron energy-loss spectroscopy [EELS]) indicated that the microstructure of LFH consisted of an amorphous LiCl-based shell surrounding a crystalline FeOCl-based core. Electrochemical measurements alongside solid-state 6,7Li nuclear magnetic resonance (NMR) and molecular dynamic simulations revealed Li+ as the sole conductive species, with a diffusion barrier of ∼0.25 eV. X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure (XAFS) results further supported interstitial Li+ diffusion at the heterointerface and within the LiCl phase, made possible by the heterointerface. Despite susceptibility to electronic conductivity, iron’s defects and multivalency (Fe³⁺, Fe²⁺) enable the Fe–O–Cl framework to accept Cl−, facilitating Li⁺ ionic conduction. A prototype solid-state cell (showing 97% Coulombic efficiency) demonstrated the viability of this heterointerface design for applications in energy storage.

通过设计和合成陶瓷异质界面实现快速离子传导
将不导电的氯化锂(Li)和氯化铁(FeOCl)结合形成[Li1+δ cl]δ+/[FeOCl]δ−异质界面复合材料(LFH),离子电导率达到>;1 mS cm−1。分析技术(扫描透射电子显微镜[STEM]和电子能量损失谱[EELS])表明,LFH的微观结构是由一个非晶的licl基壳包裹在一个结晶的feocl基核周围。电化学测量以及固态6,7Li核磁共振(NMR)和分子动力学模拟表明,Li+是唯一的导电物质,其扩散势垒为~ 0.25 eV。x射线光电子能谱(XPS)和x射线吸收精细结构(XAFS)结果进一步支持Li+在异质界面和LiCl相内的间隙扩散,这是由于异质界面的存在而成为可能的。尽管易受电导率的影响,但铁的缺陷和多价性(Fe³+、Fe²+)使Fe - o - Cl框架能够接受Cl−,从而促进Li +的离子传导。固态电池的原型(库仑效率为97%)证明了这种异质界面设计在储能应用中的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


